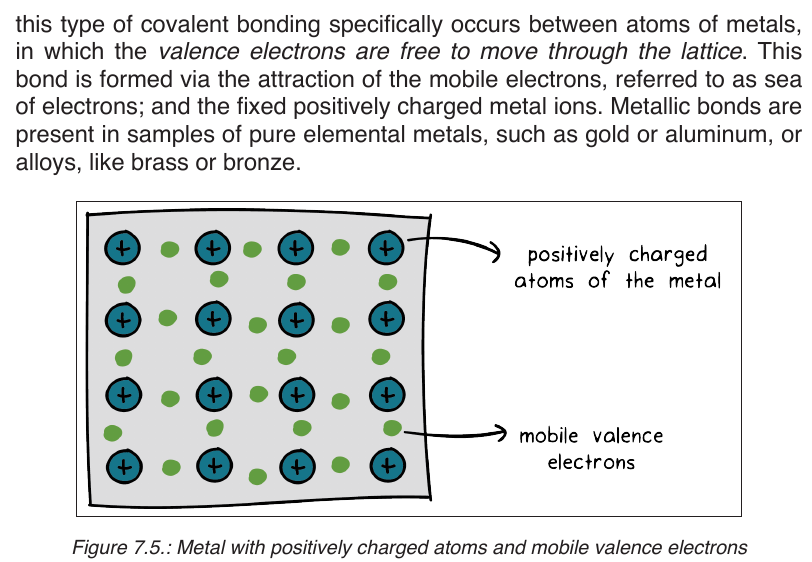
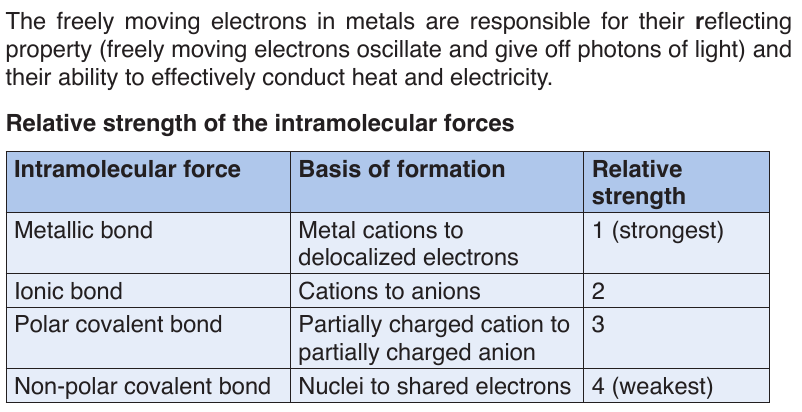
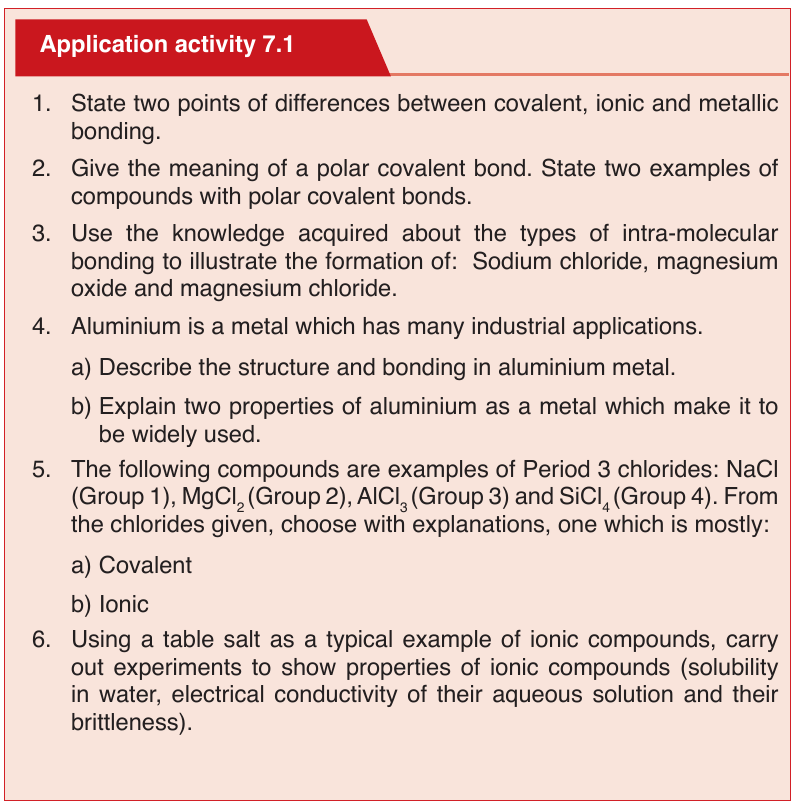
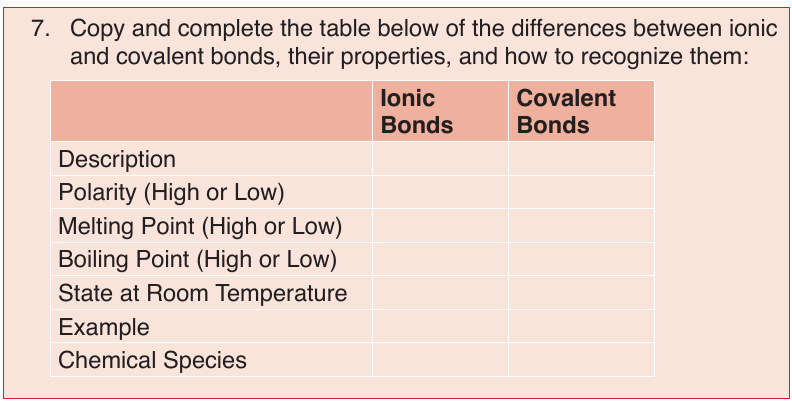
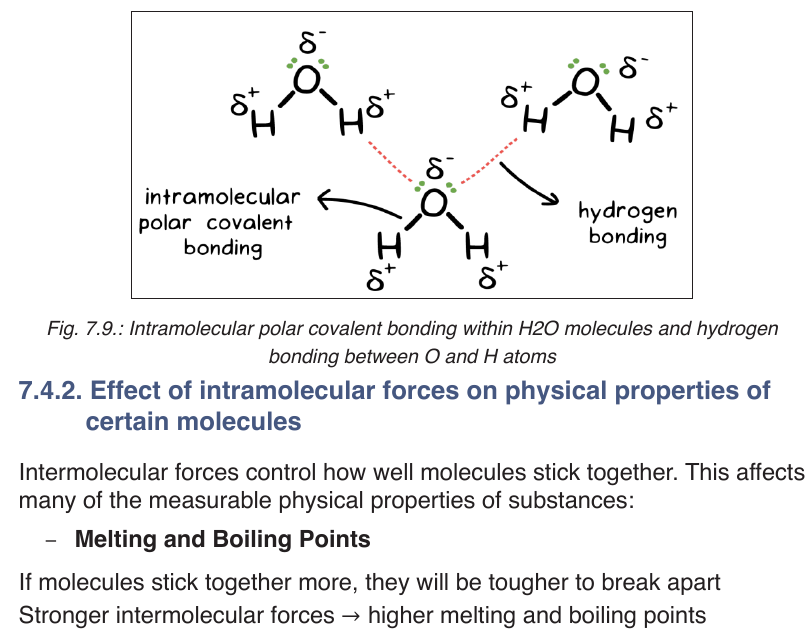
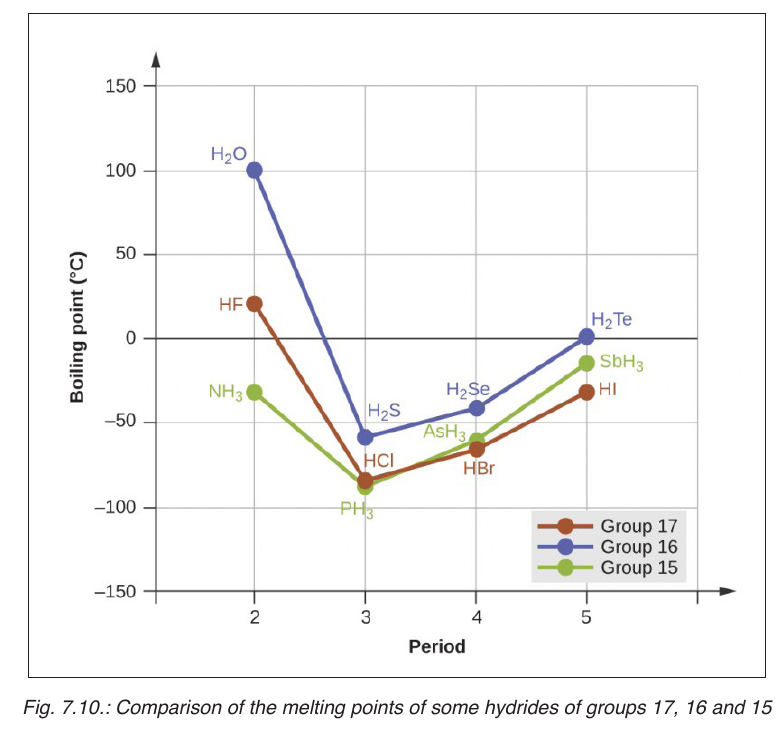
Topic outline
UNIT 1: HEALTHY NUTRITION
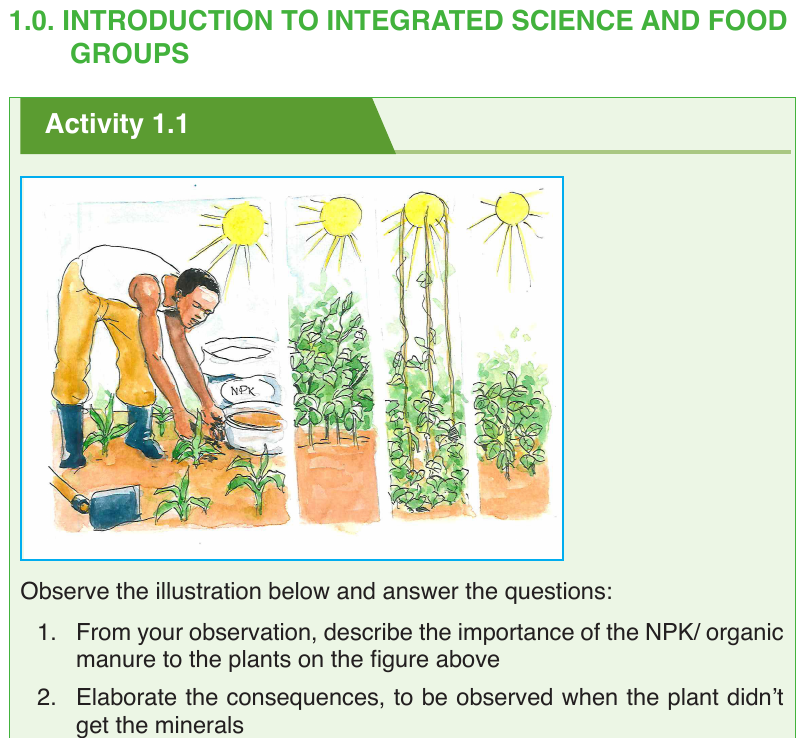
1.1. INTRODUCTION TO INTEGRATED SCIENCE
1.1.1. Definition and rationale of integrated science
human survival depends on knowledge through the exploration of the
environment. Science provides knowledge while technology provides ways
of using this knowledge. It is therefore very important to be aware of the
global dimension of science needed in our lives in order to effectively deal
with every day situation. The word “integrated” means “to restore the whole,
to come together, to be a part of, to include.” Integrated science is a subject
which incorporates the knowledge base of all the science fields, both physical
and life sciences and these science fields are included in one subject as a
whole “integrated science” in that the fields of science are not segmented.
It is a subject which offers experiences which help people to develop an
operational understanding of the structure of science that should enrich their
lives and make them more responsible citizens in the society.
Hence, integrated approach of learning science is appropriate as science
knowledge is a tool to be used by every person to effectively deal with real
world problems and life.
For examples, when you are studying digestion process of animals, you will
need the knowledge of chemical processes. Another example, in describing
the physics of light, we show how this applies to the inner workings of our
eyes, which, in turn, are sensitive to visible light in great part because of the
chemical composition of our atmosphere.
1.1.2. Interconnection between science subjects
The purpose of science is to produce useful models of reality which are used
to advance the development of technology, leading to better quality of life for
man and the environment around him.
There are many branches of science and various ways of classifying them.
One of the most common ways is to classify the branches into social sciences,
natural sciences and formal sciences.
Social sciences deal with the study of human behavior and society. Examples
of these are psychology and sociology. Natural sciences deal with the study
of natural phenomena, for example lightning, motion, and earthquakes all
which can be observed and tested.
Examples of these are physics, chemistry and biology. Formal sciences deal
with mathematical concepts and logics. An example of this is mathematics.
Note:
–– Chemistry mainly deals with the study of salts, acids and their reactions.
For a physicist to understand the working mechanism of chemical cells,
help is sought from a chemist. On the other hand, the reasons behind
the various colours observed in most of the chemical reactions are
explained by a physicist.
–– Petroleum products are dealt with by the chemist, but the transportation
of such products make use of the principles of physics.
–– In Biology, the study of living cells and small insects by a biologist
requires magnification. The concept of magnification using simple or
compound microscope is a brain child of a physicist. A good physicistneeds to have good health.
1.1.3. Relationship between sciences with other subjects
The concepts of science and other subjects might be expanded or explainable
in broader senses than you might have been exposed to, this should then
predict not only the interconnection senses already known, but should also
predict much broader interconnections. This might be useful to you and our
future civilization.
Science is about observation and experimentation of things in the physical
and natural world. If there no creative ideas, no destructive ideas, just more
ideas of the same things that exist can this be healthy? There is such a thing
as inductive reasoning not just deductive reasoning.
Now, science is the practical application of scientific knowledge. So we
could have science as a conservative subject, or we could have science as
a creative (conservative and destructive) subject, then leading to smaller or
larger sets of science.
Note:
–– In Geography, weather forecast, a geographer uses a barometer, wind gauge, etc. which are instruments developed by a physicist.
–– In Agriculture, the water sprinkler, insecticide sprayer, etc. make use of
the principles developed by physicists.
–– In History, the determination of age fossils by historians and
archaeologists use the principle developed by physicists.
–– In games and sports, accurate measurement of time, distance, mass,and others uses instruments developed by physicists.

Food nutrients include macro and micro nutrients. Macro nutrients are
needed by the body in large quantities. They include proteins, carbohydrates
and lipids while the micro nutrients are needed in small amount and they
include mineral salts and vitamins.
The foods that we eat contain different types of nutrients. It is therefore
essential that we know the components of the food that we eat in order to
live healthy lives. There are three main food groups such as: energy giving
foods, body building foods and protective foods.
Energy giving foods are necessary to provide energy for cell metabolism.
They include: carbohydrates and lipids. Some energy giving foods include
potatoes, banana, rice, maize etc.
Body building foods are needed to promote growth and tissue repair.
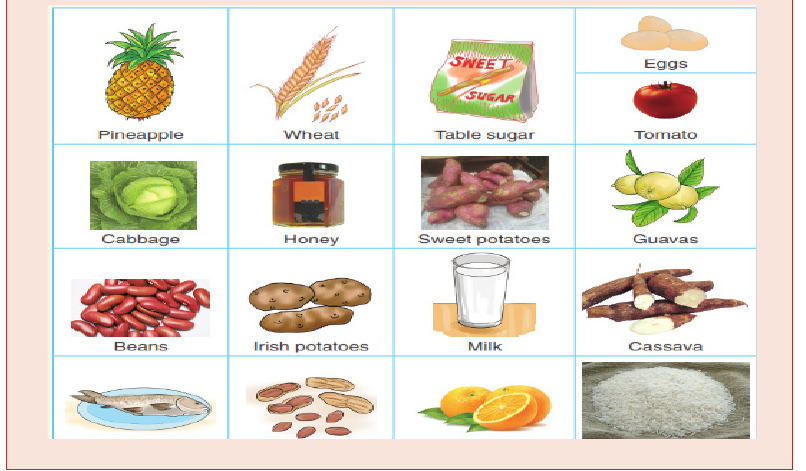
These include proteins and can be found in the meat, eggs, fish, milk, beans,
cassava leaves, etc.
Protective foods allow a good functioning of the body, and protect the body
against some deficiency diseases. They include minerals and vitamins. The
minerals can be found in fish, beans, kitchen salt and mineral water; and
vitamins are found mainly in vegetables and fruits.
Food nutrients
Food contains mainly two classes of nutrients, organic and inorganic. The
inorganic nutrients include mineral salts like calcium, phosphorous and
others like water. The organic nutrients include proteins, carbohydrates,
lipids and vitamins.
The materials that an animal’s cells require but cannot synthesize are called
essential nutrients. There are four classes of essential nutrients: essential
amino acids, essential fatty acids, vitamins, and minerals.
1.2.1. Functions of food nutrients
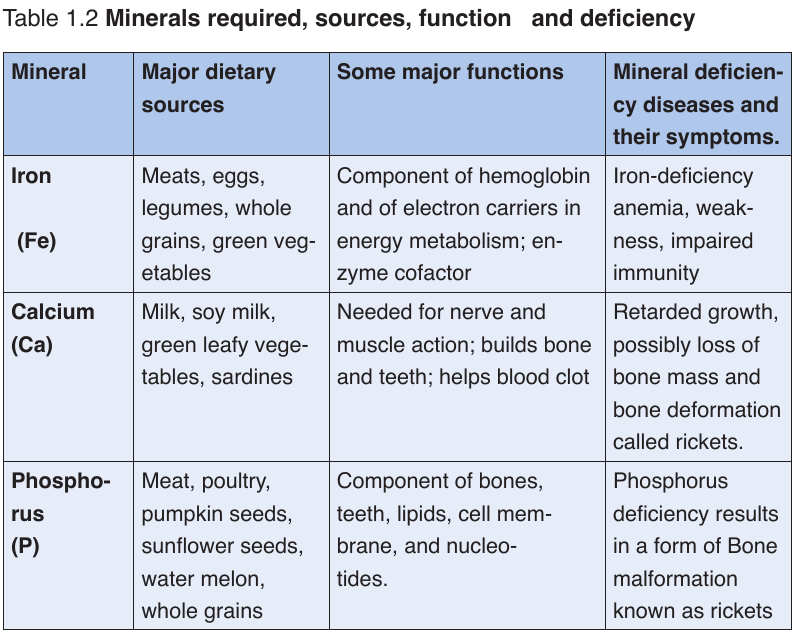
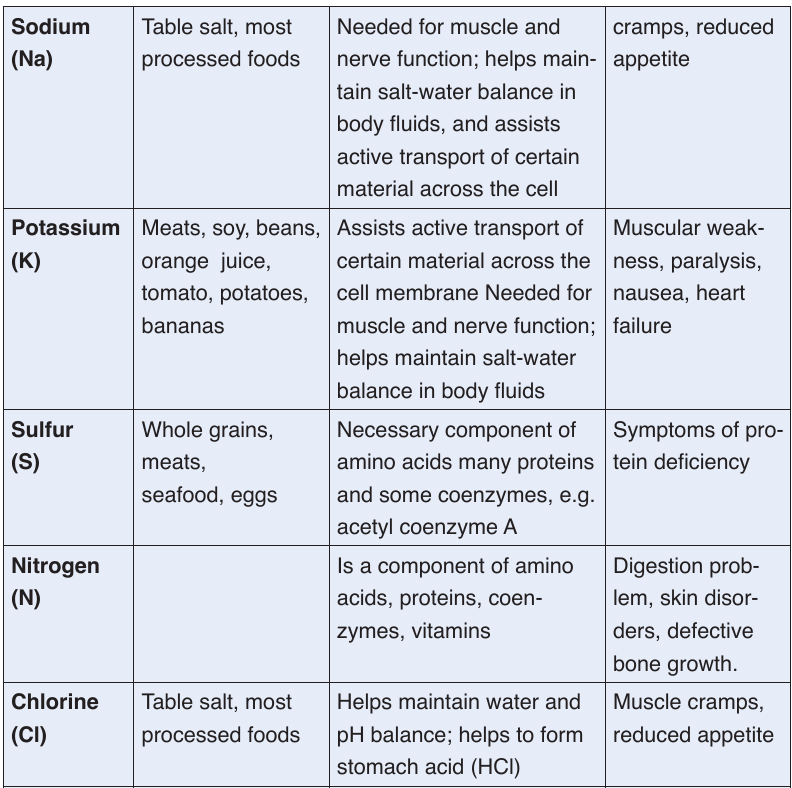
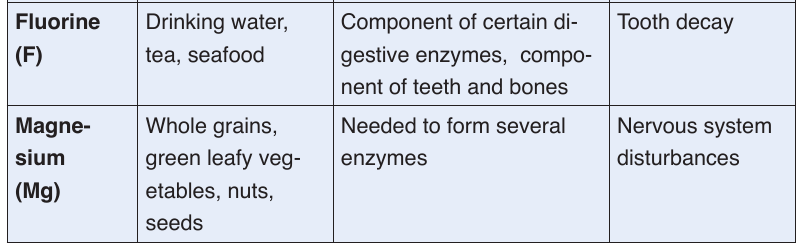
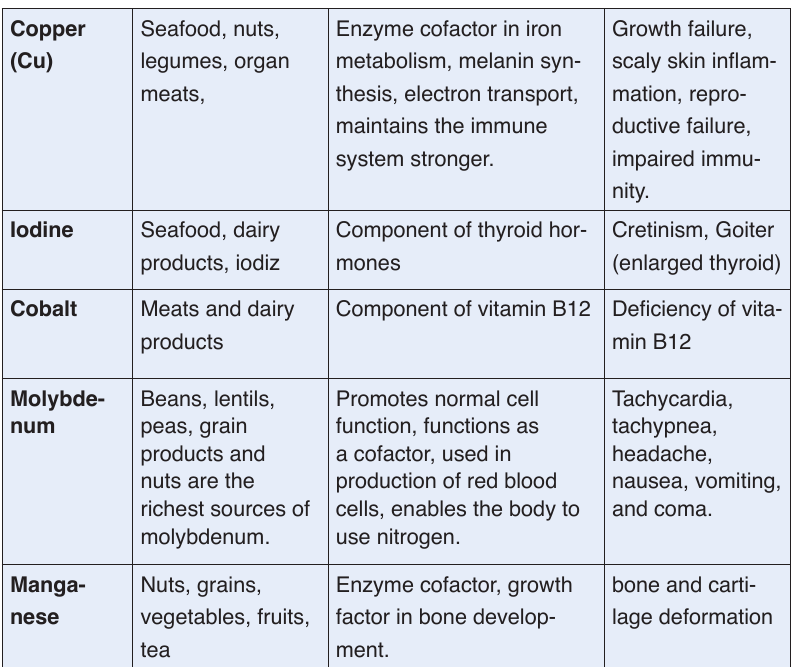
a. Minerals
Mineral are also called micronutrients because we require them in very small
quantities. They constitute about 1% of an organism by weight. Even though
they are required in a very small amount, they are nonetheless essential for
human body processes.Table 1.1: Principal minerals
Classification of minerals
The classification of minerals is based upon their requirement rather than
on their relative importance. Mineral nutrients are needed in a precisesmall amount. The five major minerals needed in human body include
calcium (Ca2+), phosphorus (H2PO4-), potassium (K+), sodium (Na +) and
magnesium (Mg2+). Mineral nutrients are grouped into two groups of mineral
salts: the macronutrients or major elements and the micronutrients or
trace elements.
Macronutrient or major elements are minerals needed by humans in a relative
large amounts (greater than 200 mg/day). Their examples include nitrogen
(NO3-), phosphorus (H2PO4-), sulfur (SO42-), calcium (Ca2+), sodium (Na+),
chlorine (Cl-), magnesium (Mg2+), and iron (Fe2+ or Fe3+). Micronutrients or
trace elements are those which are needed in minute amounts (a few parts
per million). Examples include manganese (Mn2+), iodine (I-), zinc (Zn2+),
molybdenum (MoO4-).
Human body requires mineral nutrients to survive and to carry out daily
functions and processes. Minerals keep humans healthy and have key roles
in several body functions. Humans receive minerals by eating plants that
absorb minerals from the soil and by eating meat and other products from
animals, which graze on plants. The deficiency of mineral nutrients results
into body functional disorders and diseases. Most are found in the blood and
cytoplasm of cells, where they assist basic functions. For example, calciumand potassium regulate nerve and muscle activity
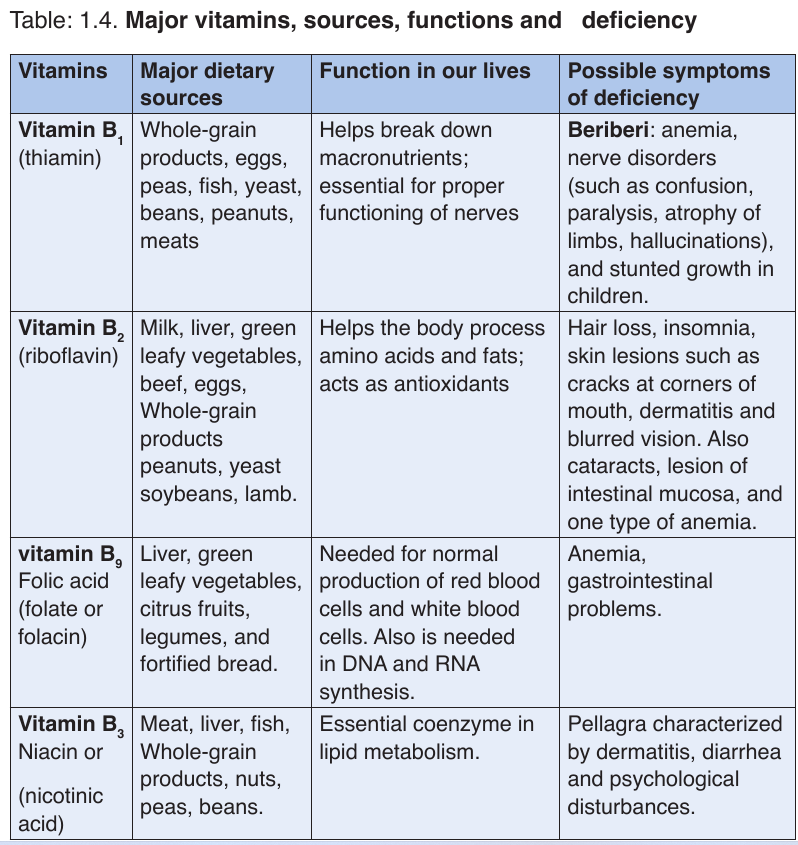
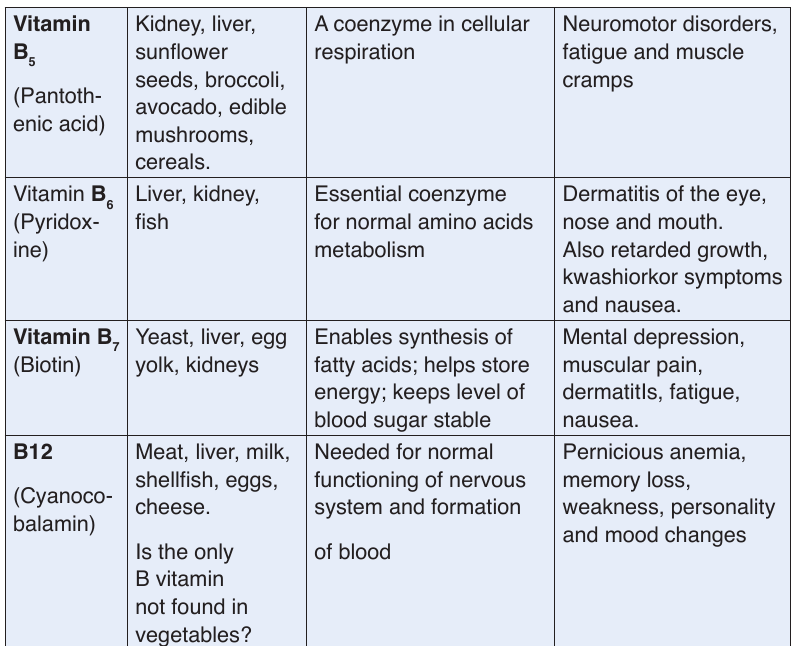
b. Vitamins
Like minerals, vitamins are also essential substances for the human body to
function properly. They are required for metabolism, protecting health and
for proper growth in children.
These are referred to as micro-nutrients. This is because our bodies require
them in very small quantities but they are very important. Depending on thevitamin, the required amount ranges from about 0.01 to 100 mg per day.
Vitamins classification
There are thirteen vitamins required by human body. They are classified by
their solubility, whether they dissolve in water or in fats.Table 1.3 Water-soluble and fat-soluble vitamins
• Water soluble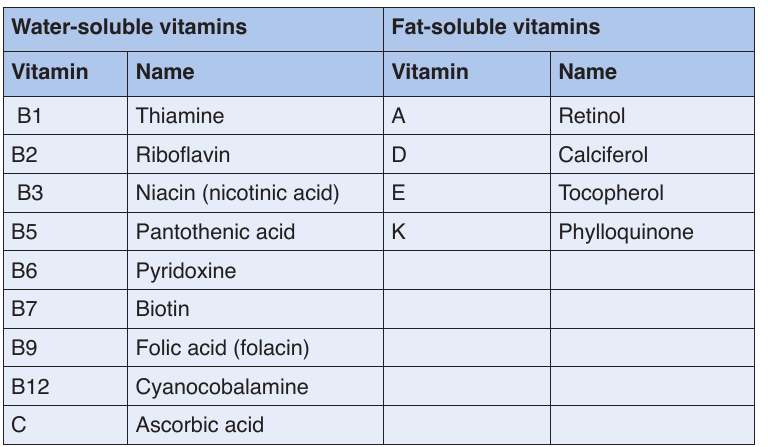
These are vitamins C and B. They are called water soluble vitamins because
they dissolve easily in water. They also dissolve when vegetables containing
these vitamins are cooked for long time.
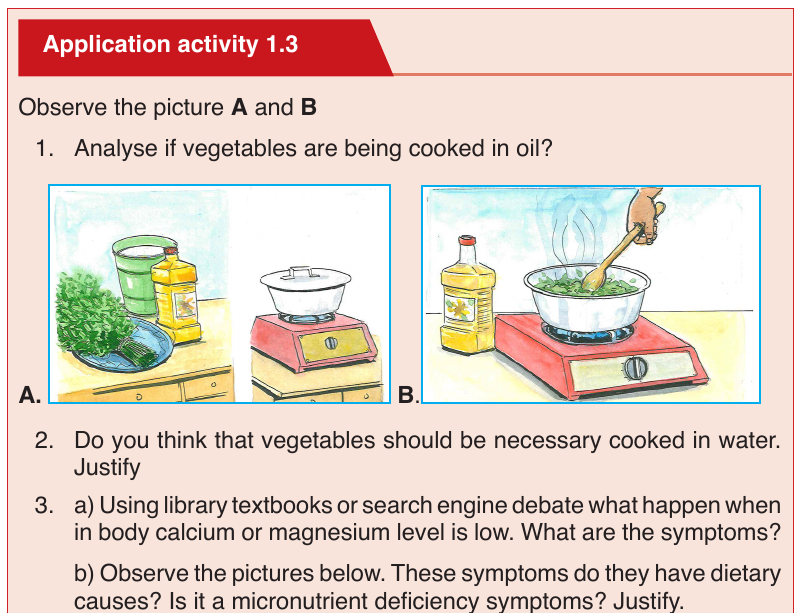
NB: We must never overcook vegetables.
• Fat soluble.
These consist of vitamins A, D, E, and K. They are called fat vitamins
because they dissolve easily in oil and fat.
NB: We fry vegetables in some oil to be able to benefit from vitamin A, D, E,
and K in them. If we only oil boil or steam them, our bodies will not be able
to extract the vitamin in vegetables.
The vitamins are required for metabolism, protecting health and for proper
growth in children. Vitamins also assist the formation of hormones, blood
cells and genetic material.
Vitamins require no digestion and are absorbed directly from the smallintestine into the blood stream. Features shared by all vitamins:
–– They are not digested or broken down for energy
–– They are not synthesized into the body structures (are essential)
–– Most are rapidly destroyed by heat.
–– They are essential for good human health (needed in a very small amount)
–– They are required for chemical reactions in cells, working in association with enzymes.
Like minerals, vitamins are also essential substances for the human body to
function properly. They are required for metabolism, protecting health andfor proper growth in children.

c. Carbohydrates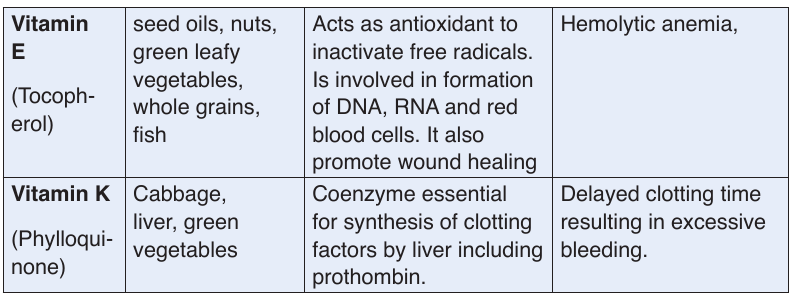
They are macronutrients that provide our bodies with energy and warmth.
The word carbohydrate suggests that these organic compounds are
hydrates of carbon. Their general formula is Cx (H2O)y . The general function
of carbohydrates is to provide energy that is used in cellular metabolism.
Carbohydrates are divided into three groups including the monosaccharides
(single sugars: glucose, fructose and galactose), disaccharides (double
sugars: sucrose, maltose and lactose) and polysaccharides (many sugars:
starch, glycogen and cellulose).
NB: We need carbohydrates to do work also to keep our bodies warm.Table 1.5: Types of disaccharides and their monomers
The carbohydrates are energy giving nutrients. They are burned by Oxygen in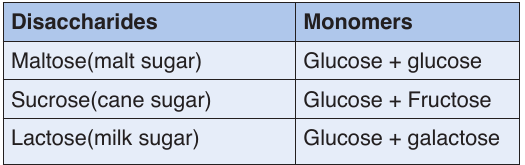
a process of cell respiration to produce energy to be used in cell metabolism.
The common know monosaccharides of carbohydrates is glucose with
molecular formula C6H12O6. If is burned by Oxygen, it produces energy as
shown in equation of cell respiration below: C6H12O6 +6 O26 CO2 +6 H2O+
Energy (ATP + heat).
All monosaccharides and disaccharides have the following characteristics:
sweet taste, soluble in water and lower molecular mass. In the same way
that two monosaccharides may combine in pairs to give a disaccharide,
many monosaccharides may also combine by condensation reactions to
form a polysaccharide. The polysaccharides like starch are not soluble inwater, and do not have the sweet taste.
d. Lipids (Fats and oils)
Fat sometimes ‘lipids’ refers to both fats and oils. Where by fats and oils
have the same basic chemical structure but their appearance differs at room
temperature that is, fats are solids at room temperature while oils are liquids
at room temperature. Fat is composed of three elements which are carbon,oxygen and hydrogen.
Sources and classification of lipids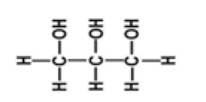
Fats and oils are obtained from both the plants and animals. And fat is
present in food either as visible fat or invisible fat.
Visible fat is the one that is easily seen or detected in food for example; fatin meat, butter, margarine, lard, suet and cooking fat and oil.
Invisible fat is the part of food that is not easily seen for example fat with
in lean meat, egg yolk, flesh of oily fish, groundnuts, soya beans, avocado
and fat found in prepared foods, for example, pastry, cakes, biscuits, Frenchfries, pancakes, croquettes.
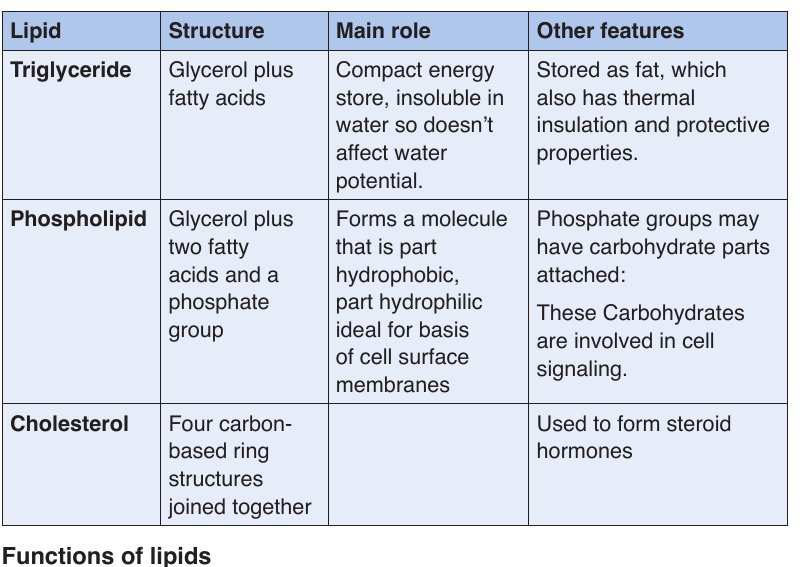
Lipids are of different types as it is summarized in the following table
Table 1.6: Lipids, structure, main role and features
e. Proteins
These are also referred to as macro-nutrients. The protein are also called
body- building food.
Proteins are made of complex molecules which contain elements like oxygen,
hydrogen, carbon, nitrogen and sometimes Sulphur and phosphorous. The
protein molecules are made up of small units calledAmino acids joined together like links in a chain.
There are 21 different amino acids and each has its own chemical name.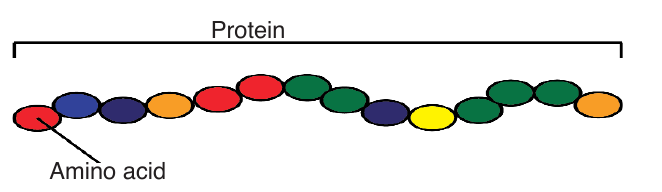
Different proteins are made when different numbers and types of amino acids
combine through a covalent peptide bond. Proteins are therefore known as
polypeptides.
Examples of proteins:
a) Collagen, myosin and elastin found in meat,
b) Caseinogen, lactalbumin, lacto globulin found in milk,
c) Avalbumin, mucin and liporitellin found in eggs,
d) Zein found in maizeThe 21 different amino acids found in protein are:
They are used to repair, to build, to maintain our bodies; to make muscles
and to make breast milk during lactation period. The proteins are classified
into two categories: animal or complete proteins and plant proteins orincomplete proteins.
Functions of proteins
Proteins are large organic compounds formed by amino acids and they are
not truly soluble in water. In addition to carbon, hydrogen and oxygen, proteinsalways contain nitrogen, usually Sulphur and sometimes phosphorus.

1.2.2. Balanced diet and food service techniques
a. Balance diet
Eating a balanced diet means eat at least 5 portions of a variety of fruit
and vegetables every day (see 5 A Day), base meals on higher fibre starchy
foods like potatoes, bread, rice or pasta, have some dairy or dairy alternatives
(such as soya drinks) eat some beans, pulses, fish, eggs, meat and other
protein
Food variety means eating a wide variety of foods from each of the five food
groups, in the amounts recommended. Eating many different foods helps
maintain a healthy and interesting diet which provides a range of different
nutrients to the body. Eating a variety of foods promotes good health and
can help reduce the risk of disease. ( https://www.google.balanced diet chart
for family)
The nutritional requirement is influenced by age, sex, growth, pregnancy
and breastfeeding, illness, psychological and emotional stress, activity
level and other factors like smoking and drinking. Biological factors include
age, gender, growth, disease states, and genetic makeup. Among the no
biological factors, socio-economic status is the most important. Poverty is
one of the major socio-economic causes of variation in nutrient intake, and it
also impacts nutrient requirements. ( https://www.google.balanced diet chart
for family)
Example:
Aging is linked to a variety of changes in the body, including muscle loss,
thinner skin and less stomach acid. ... Low stomach acid can affect the
absorption of nutrients, such as vitamin B12, calcium, iron and magnesium.
Although the recommended breakdown of carbohydrate, protein, and fat are
the same for both genders, because men generally need more calories, they
also require higher total intake of each of the macronutrients. Women need
fewer calories than men, but in many cases, they have higher vitamin and
mineral needs.
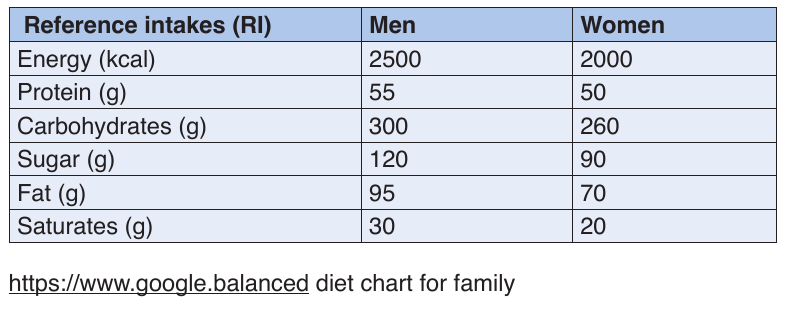
Reference Intakes
Nutritional needs vary depending on sex, size, age and activity levels so use
this chart as a general guide only. The chart shows the Reference Intakes
(RI) or daily amounts recommended for an average, moderately active
adult to achieve a healthy, balanced diet for maintaining rather than losing or
gaining weight. The RIs for fat, saturates, sugars and salt are all maximum
amounts, while those for carbs and protein are figures you should aim to
meet each day. There is no RI for fibre although health experts suggest wehave 30g a day.
A balanced diet is one that contains all nutrients required in health in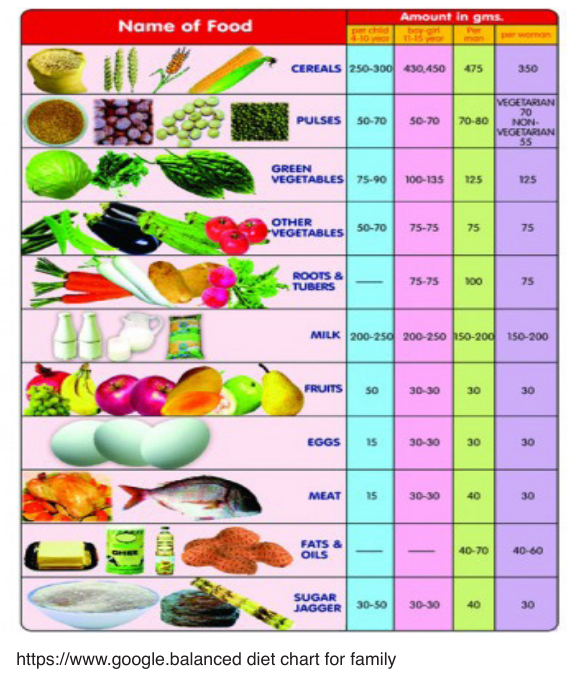
appropriate proportion. A balanced diet must should contain all food groups
such as: body building food, energy giving food and protective food in an
appropriate amount. A balanced diet help a person to:
–– Make you strong
–– Provide better health
–– Make you more productive–– Ensure strong immune system
It’s not hard to include foods from the five food groups into appetizers and
meals. Some suggestions include:
–– Vegetables and legumes – raw or cooked vegetables can be used as
a snack food or as a part of lunch and dinner. Salad vegetables can be
used as a sandwich filling. Vegetable soup can make a healthy lunch.
Stir-fries, vegetable patties and vegetable curries make nutritious
evening meals. Try raw vegetables like carrot and celery sticks for a
snack ‘on the run’.
–– Fruit – this is easy to carry as a snack and can be included in most
meals. For example, try a banana with your breakfast cereal, an apple
for morning tea and add some berries in your yoghurt for an afternoon
snack. Fresh whole fruit is recommended over fruit juice and dried fruit.
Fruit juice contains less fibre than fresh fruit and both fruit juice and
dried fruit, and are more concentrated sources of sugar and energy.
Dried fruit can also stick to teeth, which can increase the risk of dental
caries.
–– Bread, cereals, rice, pasta and noodles – add rice, pasta or noodles
to serves of protein and vegetables for an all-round meal. There are
many varieties of these to try. Where possible, try to use wholegrains
in breads and cereals.
–– Lean meat, fish, poultry, eggs, nuts, legumes and tofu – these can
all provide protein. It’s easy to include a mixture of protein into snacks
and meals. Try adding lean meat to your sandwich or have a handful
of nuts as a snack. You can also add legumes to soups or stews for an
evening meal.
–– Milk, yoghurt and cheese – try adding yogurt to breakfast cereal
with milk, or using cottage cheese as a sandwich filling. Shavings of
parmesan or cheddar can be used to top steamed vegetables or a
salad. Use mostly reduced fat products.
Feeding on unbalanced diet for a longtime may lead to malnutritional
diseases. Malnutrition means feeding on a meal lacking some food nutrients
(deficient diseases), or on a meal with all food nutrients but in unappropriated
amount (over eating).
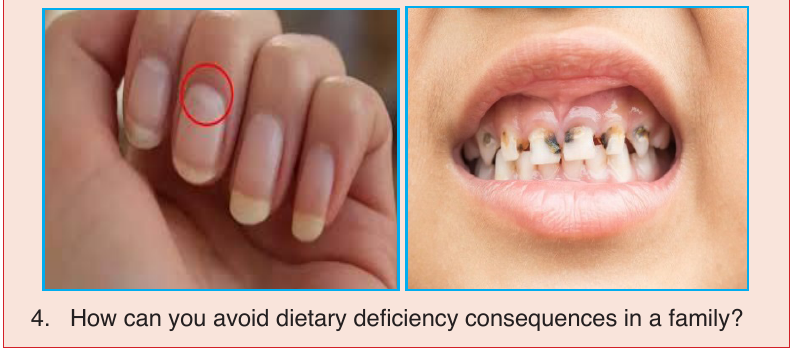
Some deficient diseases include: kwashiorkor (caused by the meal lacking
proteins), marasmus (caused by the meal lacking overall nutrients), and
goitre (caused by the meal lacking iodine). The diseases caused by over
eating include: obesity, a condition in which excessive fats are deposited
in the body. More malnutritional diseases are described in the tables abovedescribing functions of minerals and vitamins.
b. Basic food service technics
They are many different approaches of serving food. An operation should use
a service style that is the best to satisfy its family members. The traditional
table service provides service for family members who are seated at table.
The English service comparable to Rwandan style is a type of service known
as “family style service”. In this service the big dish is placed in front of thehost along with serving plate and family members serve themselves.
• Principles for meal service
The family style meal service allows participants to eat together and to
make food choices based on individual appetites and food preferences. It
promotes mealtime as a learning experience to help participants develop
positive attitudes toward nutritious foods, share in group eating situations,
and develop good eating habits. Family style meal service can be conducted
in a variety of ways. For example, participants may help in preparing for the
meal by clearing the table and setting places, sharing conversation during
the meal, and cleaning up after the meal.
Family style meal service operates as follows:
–– All required meal components are placed on the table at the same time.
–– Participants may serve themselves from serving dishes that are on the table
–– Adults supervising the meal help those participants who are not able to serve themselves.
–– Participants can make choices selecting foods and in the size of the serving.
–– A supervising adult actively encourage family members to serve
themselves and offers the food item again later in the meal if member
(s) initially refuse the food or take a very small portion. Adult should
model good eating habits while supervising participants at the dining
table.
• Standards of serving food temperatures
The importance of temperature
The crucial important part of food safety in the home is to keep hot food hot
and to keep cold food cold. For safety it is vitally important to keep food out
of that danger zone.
The food being served should be kept at specified range and appropriate
temperature (T):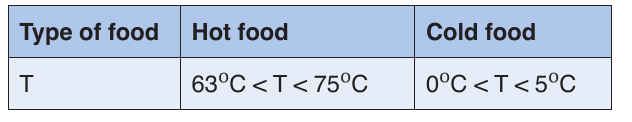
Note:
–– The temperature danger zone for bacteria reproduction and growth: 5OC < T < 63OC, food is not suitable for eating.
–– Bacteria do not multiply and start to die at 63OC above and do not grow and multiply at 5OC below.
End unit assessment
1. Analyze the importance of food nutrients.
2. Explain the importance of a balanced diet.
3. Explain how the condition factors (age, gender; activity; pregnant and breastfeeding mothers) affect the dietary needs of humans.
4. Justify the different functions of food nutrients in the body.
5. Classify vitamins and minerals as nutrients.
6. Organize the diet components in food groups.
7. Organize a list of foods that are good sources of specific food nutrients.
8. Prepare a balanced diet
9. Discuss services techniques of healthy diet.10. Recognize the role of integrated science in everyday life experiences
Files: 2UNIT 2: STRUCTURE ELECTRONIC OF CONFIGURATION AN ATOM AND

The ancient Greek philosophers Leucippus and Democritus believed that
atoms existed, but they had no idea as to their nature. Centuries later, in
1803, the English chemist John Dalton, guided by the experimental fact that
chemical elements can’t be decomposed chemically, was led to formulate
his atomic theory.
Dalton’s atomic theory was based on the assumption that atoms are
tiny indivisible entities, with each chemical element consisting of its own
characteristic atoms.
1) Dalton’s Atomic Theory
a. Each element is made up of tiny particles called atoms.
b. The atoms of a given element are identical; the atoms of different elements are different in some fundamental way(s).
c. Chemical compounds are formed when atoms of different elements combine with each other. A given compound always has the same relative numbers and types of atoms.
d. Chemical reactions involve reorganization of the atoms—changes in the way they are bound together. The atoms themselves are not changed in a chemical reaction.
e. Dalton’s atomic theory successfully explained the following laws –
conservation of mass, constant composition and multiple proportions.
However, it failed to explain certain other observations like the
generation of electricity on rubbing glass or ebonite with silk or fur.
These observations propelled the discovery of sub-atomic particles
in the 20th century. Let’s learn about the discovery of the first sub-atomic particle – Electron.
The atom is now known to consist of three primary particles: protons,
neutrons, and electrons, which make up the atoms of all matter.
A series of experimental facts established the validity of the model.
Radioactivity played an important part. Marie Curie suggested, in 1899,
that when atoms disintegrate, they contradict Dalton’s idea that atoms are
indivisible. There must then be something smaller than the atom (subatomic
particles) of which atoms were composed.
Long before that, Michael Faraday’s electrolysis experiments and laws
suggested that, just as an atom is the fundamental particle of an element, a
fundamental particle for electricity must exist. The “particle” of electricity was given the name electron.
a. Discovery of the electron
Experiments conducted by the British physicist Joseph John Thomson, in
1897 proved the existence of the electron and obtained the charge-to- mass
ratio for it.
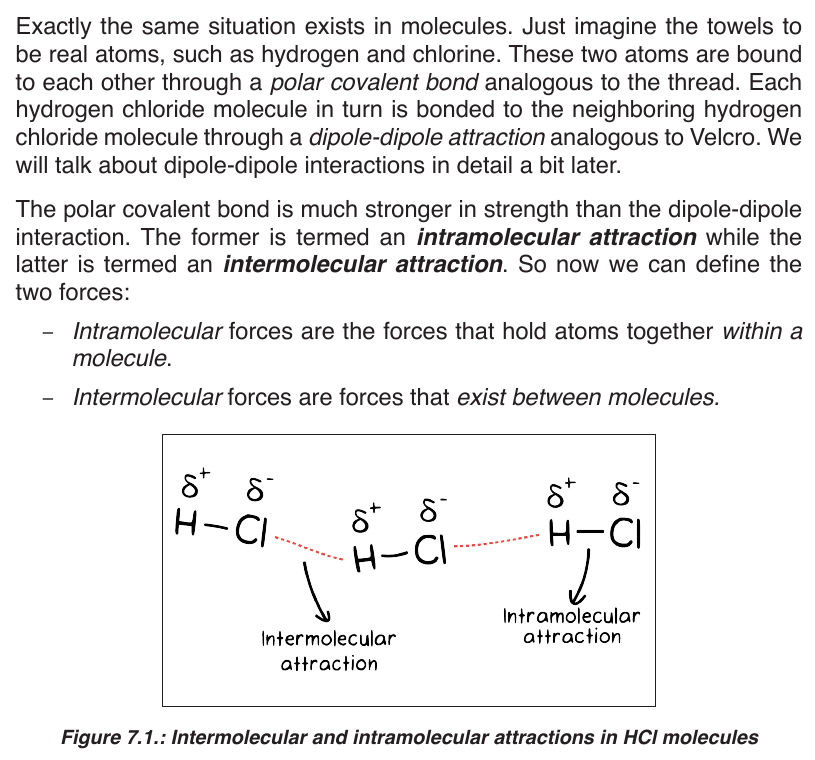
Conclusions from the Study of the Electron:
–– All elements must contain identically charged electrons. Concluded that electron was part of an atom.
–– Atoms are neutral, so there must be positive particles in the atom to balance the negative charge of the electrons
–– Electrons have so little mass that atoms must contain other particles that account for most of the mass
Thomson believed that the electrons were like plums embedded in a
positively charged “pudding,” and thus his atomic model was called the
“plum pudding” model.
Efforts were then turned to measuring the charge on the electron, and these
were eventually successful and in 1916 – Robert Millikan determines the
mass of the electron: 1/1840 the mass of a hydrogen atom. The electron has
a mass of 9.11 x 10-28 g and has one unit of negative charge
b. Discovery of the nucleus, 1911
In 1911, Ernest Rutherford (1871-1937) and his co-workers discovered the
nucleus and their main conclusions were the following.
–– The nucleus is small
–– The nucleus is dense
–– The nucleus is positively charged and electrons are distributed around the nucleus and occupy the most of the volume.
The positively charged particles in the nucleus were called protons. The
Rutherford Atomic Model was called a “nuclear model”
Neils Bohr worked under Rutherford but found problems with his theory.
He ultimately determined that electrons are in circular orbits with increasingenergy levels.
c. Discovery of the neutrons, 1932
In spite of the success of Rutherford and his co-workers in explaining atomic
structure, one major problem remains unsolved.
If the hydrogen contains one proton and the helium atom contains two
protons, the relative atomic mass of helium should be twice that of hydrogen.
However, the relative atomic mass of helium is four and not two.
James Chadwick, English physicist (1891-1974), showed that the origin of
the extra mass of helium was due to uncharged particles present in the
nucleus that they call neutrons.
Bohr’s theory said that the protons are in the middle and the electrons travel
in specific energy levels and orbits around the nucleus
The modern model is basically the same except the nucleus contains protons
and neutrons
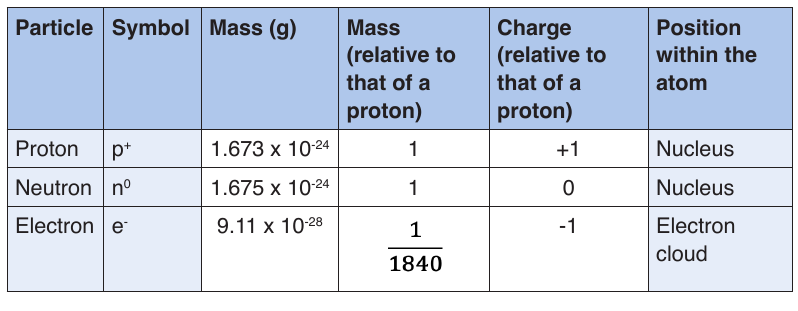
2) Properties of sub-atomic particles
The following table summarizes the relative masses, the relative chargesand the position within the atom of these sub-atomic particles.
a. Atomic number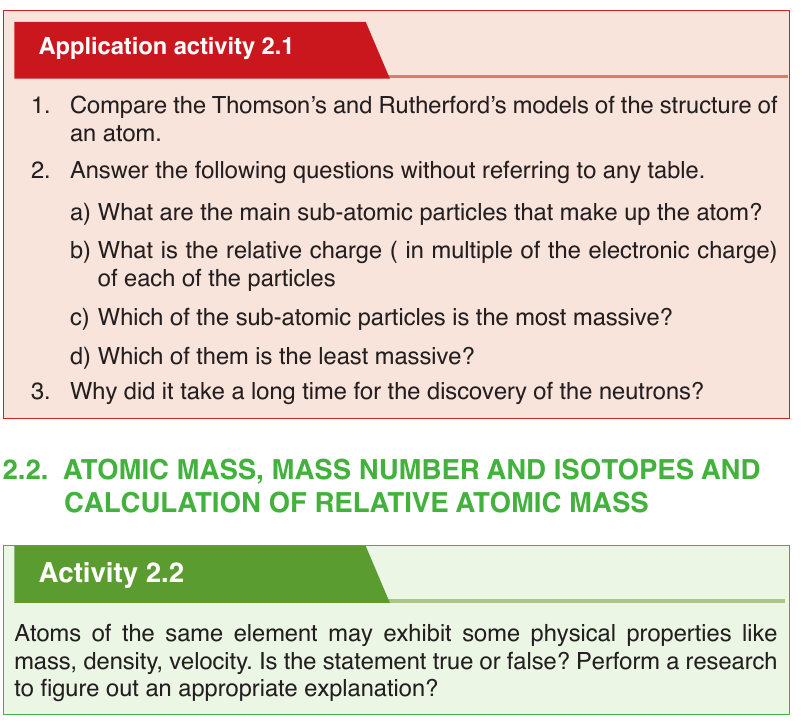
The atomic number (Z) or proton number is the number of protons in the
nucleus of an atom. It corresponds to the order of the element in the periodic
table.
The number of the protons in the nucleus of an atom determines the element
to which the atom belongs. If an atom has an atomic number of 7, the atom
must be a nitrogen atom. All nitrogen atoms have 7 protons in the nucleus.
Atoms carry no overall charge. The number of protons must therefore be the
same as the number of electrons.
b. Mass number
The mass number (A) or nucleon number is the sum of the number of
protons and the number of neutrons in the nucleus of an atom.
The number of neutrons can be obtained by subtracting the atomic number
from the mass number.
Chemists use the following shorthand to represent an atom. The mass
number is shown as a superscript (top number) and the atomic number is
shown as a subscript (bottom number) beside the symbol of the element.Example:
Each fluorine atom contains: 9 protons, 9 electrons and 10 neutrons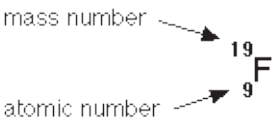
The term nuclide is used to describe any atomic species of which the
proton number and the nucleon number are specified. The species and are
nuclides.
c. Isotopes
Isotopes are atoms of the same element with the same atomic number but
different mass numbers. They have different numbers of neutrons. They are
nuclides of the same element.
Example: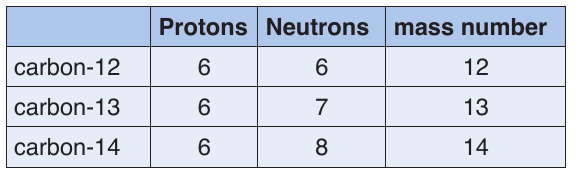
Isotopes of an element have the same chemical properties because they
have the same number of electrons.
When elements react, it is the electrons that are involved in the reactions.
This means that the isotopes of an element cannot be differentiated by
chemical reactions.
Because isotopes of an element have different numbers of neutrons,
they have different masses, and isotopes have slightly different physical
properties.
Isotopes and their abundance are estimated using an apparatus called massspectrometer (See figure 2.1)
The relative isotopic masses of all others atoms are obtained by comparison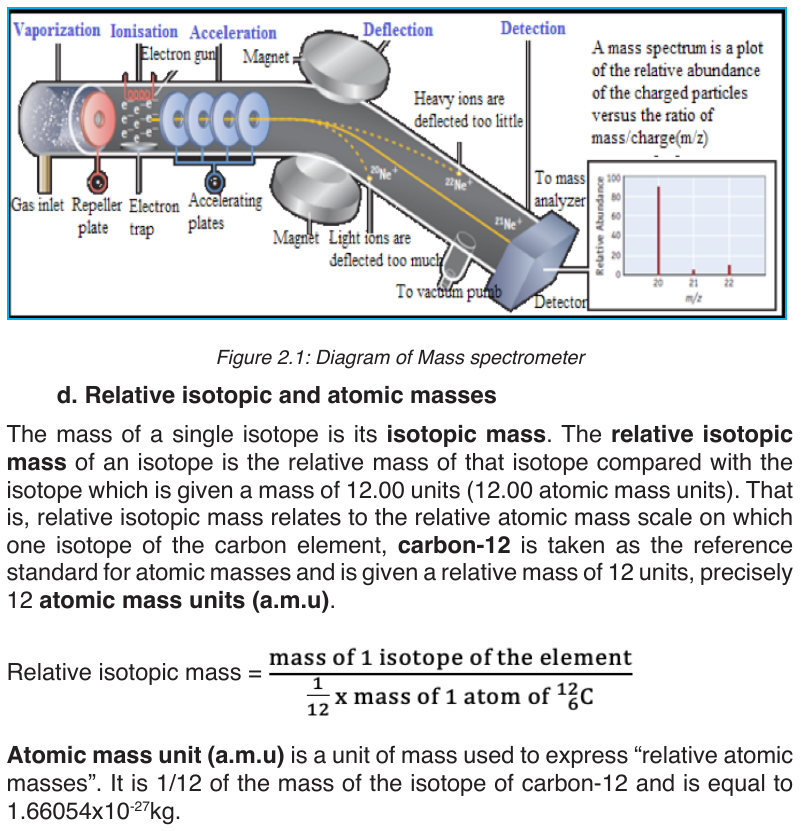
with the mass of a carbon-12 atom.
On that scale, the relative atomic mass of a proton and that of a neutron are
both very close to one unit (1.0074 and 1.0089 units respectively). Since
the relative mass of an electron is negligible (0.0005units), it follows that all
isotopic masses will be close to whole numbers.
However relative atomic masses of elements are not close to whole numbers
because natural occurring elements are often mixtures of isotopes.
The relative atomic mass (RAM) of an element, Ar , is the average of the
relative isotopic masses of the different isotopes weighted in the proportionsin which they occur.
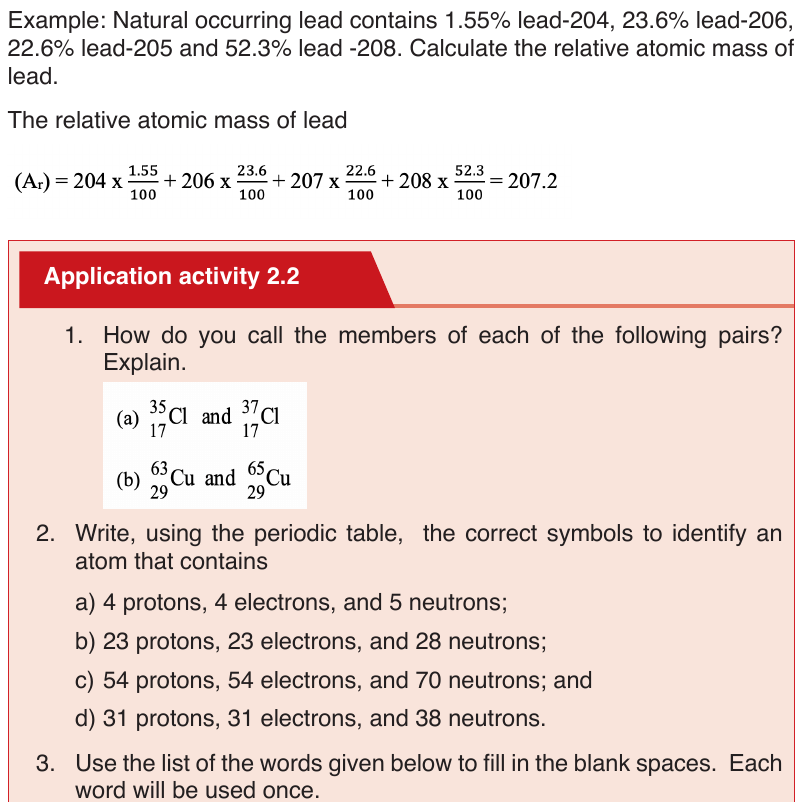
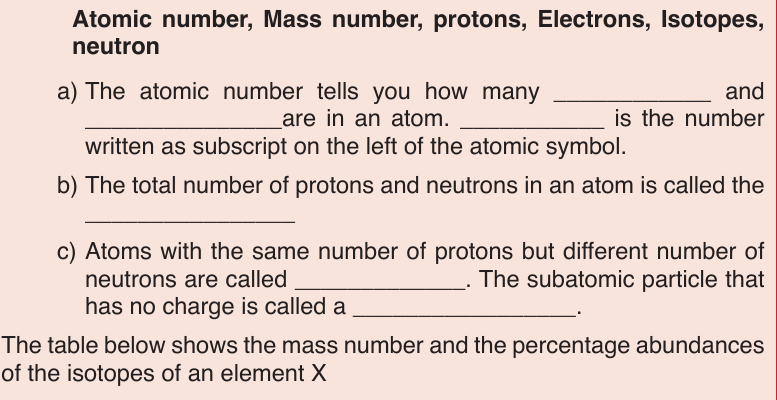
The Electron Configuration is the way electrons are arranged around the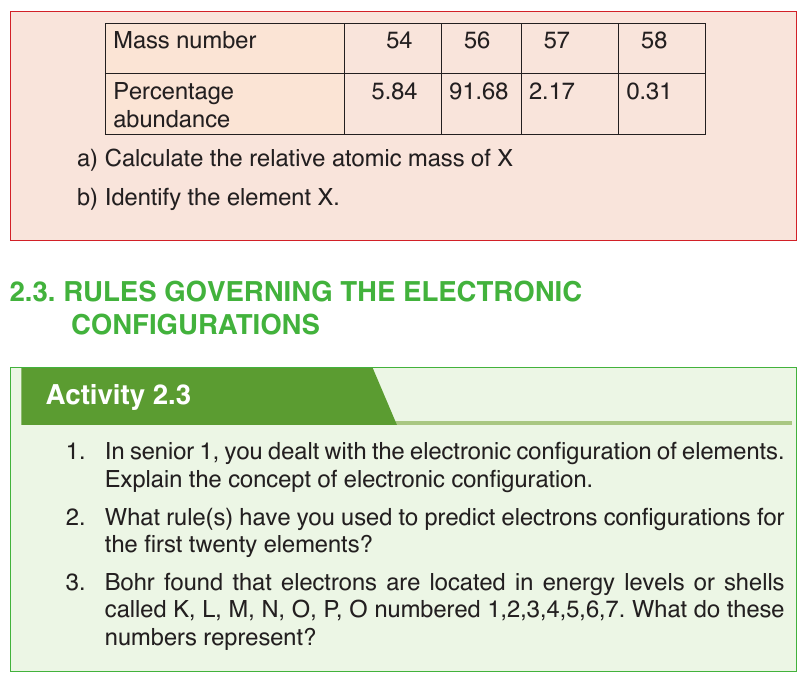
nucleus. Electrons occupy shells starting with the one closer to the nucleus,
i.e by increasing energy level. Energy levels are numbered 1, 2, 3, 4, 5, 6, 7
starting with K. Each of these numbers is called energy quantum number or
principal quantum numbers.
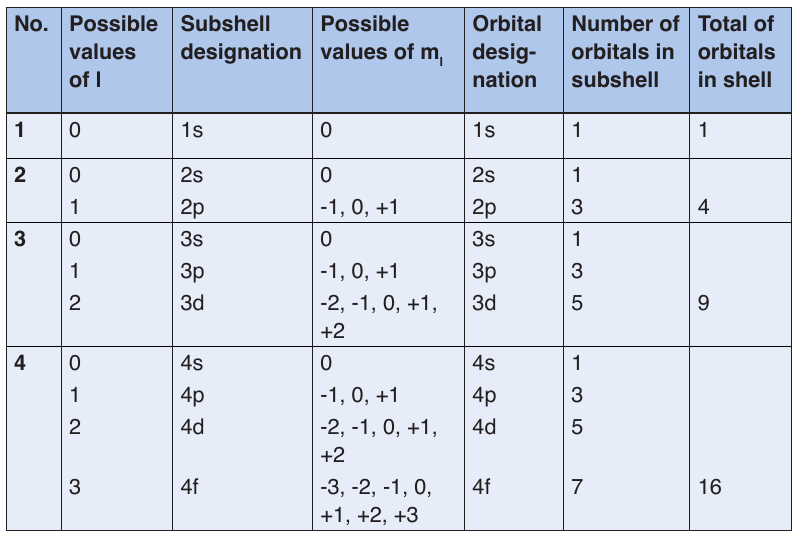
a. Quantum numbers
Energy levels or shells are subdivided into sub-shells known as s, p, d, f.
Each sub-level is split into orbitals. Orbitals of a given sub-shell have the same
name. Each electron is associated with a set of four quantum numbers s
The principal quantum number, n, can have positive integral values 1, 2,
3, 4,.... It governs the energy of the electron and also its probable distance
from the nucleus. The most stable electronic state of an atom is called its
ground state. Any higher energy state is called excited state.
The angular momentum quantum number or (azimuthal quantum
number), l, can have an integral values from zero to (n-1) for each value
of n. It determines the shape of the volume of space that an electron canoccupy. It also indicates the number of sub-levels for each level.
The values of l is generally designated by the letters:
If an electron has a principal quantum number n=2 and an angular momentum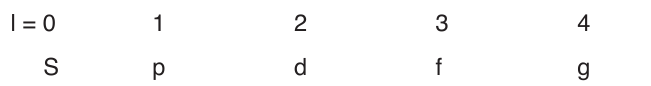
quantum number l=0 it is said to be a 2s electron.
–– The magnetic quantum number, ml, has values ranging from –l to +l.
Within a sub- shell, the value of ml depends on the value of the angular
momentum quantum number, l. For a certain value of l, there are (2l +
1) integral values of ml as follows: -l, (-l +1), . . . 0, . . . (+l-1), +l.
It determines the spatial orientation of an orbital.
–– The (Electron) Spin Quantum Number, ms, may have values of - 1⁄2
or + 1⁄2 only. The value of ms does not depend on the value of any other
quantum number. It represents the spin of an electron that occupies a
given orbital. Electrons will spin opposite each other in the same orbitalTable 2.1: Relationship among values of n, l, ml through n=4
–– Atoms of the various elements differ from each other in their values of Z and electrons.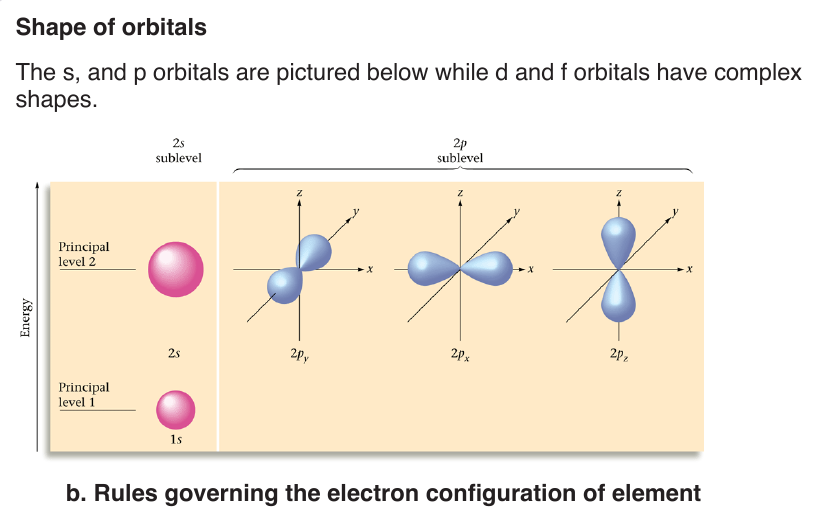
–– Electrons in atoms are arranged in orbitals and shells.
–– Orbitals are characterized by the quantum numbers n, l and ml.
–– Orbitals having the same value of n are said to be in the same shell. Orbitals having the same values of n and l are said to be in the same subshell.
–– Electrons are distributed in orbitals following the rules below.
b. 1 Pauli Exclusion Principle
No two electrons in the same atom can have the same set of the four quantum
numbers. If two electrons have the same values of n, l, ml, they must have
different values of ms. Then, since only two values of ms are allowed, an
orbital can hold only two electrons, and they must have opposite spins.
b. 2 Hunds’ rule
Electrons occupy all the orbitals of a given sublevel singly before pairing
begins.
Spins of electrons in different incomplete orbitals are parallel in the ground
state. The most stable arrangement of electrons in the subshells is the onewith the greatest number of parallel spins.
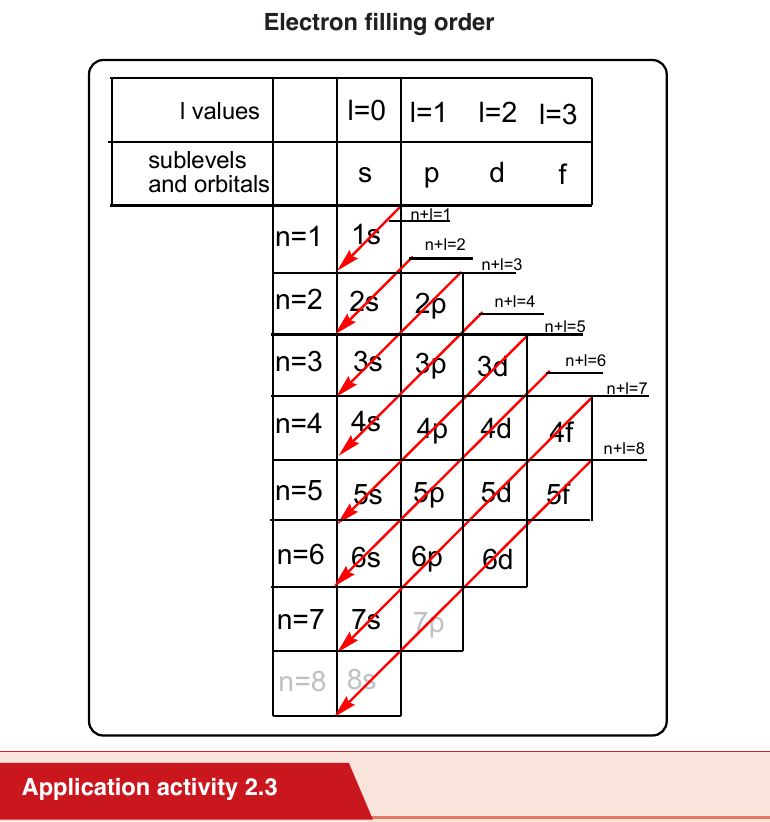
b. 3 Aufbau principle or build up principle or construction principle
The Aufbau principle or build up principle or construction principle state that
“Electrons fill lower energy orbitals (closer to the nucleus) before they fillhigher energy ones”.


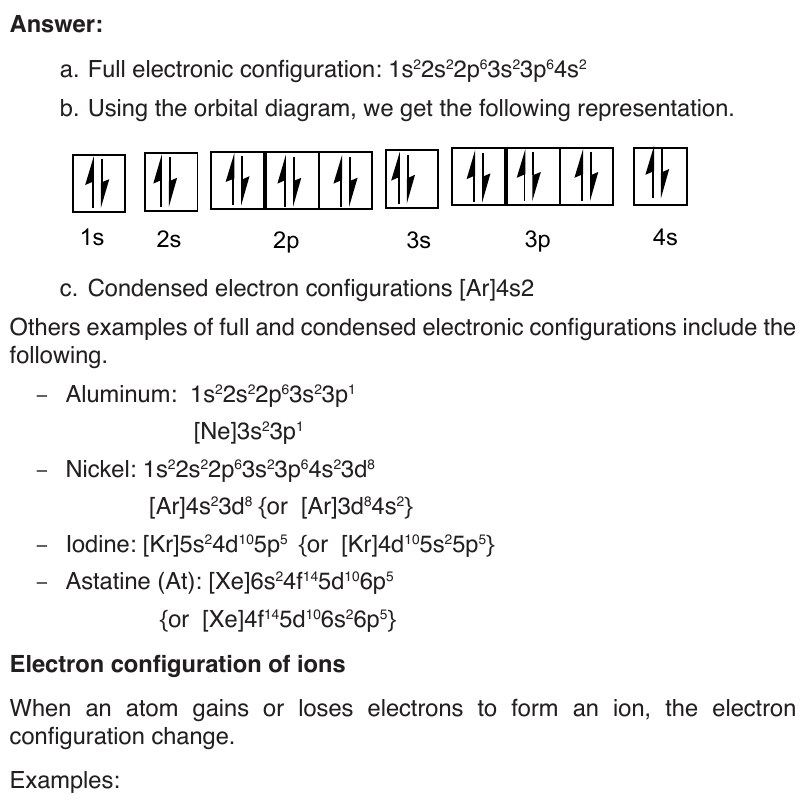
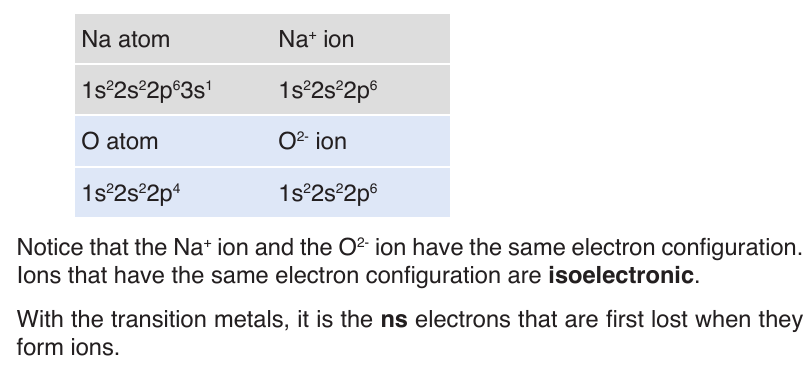
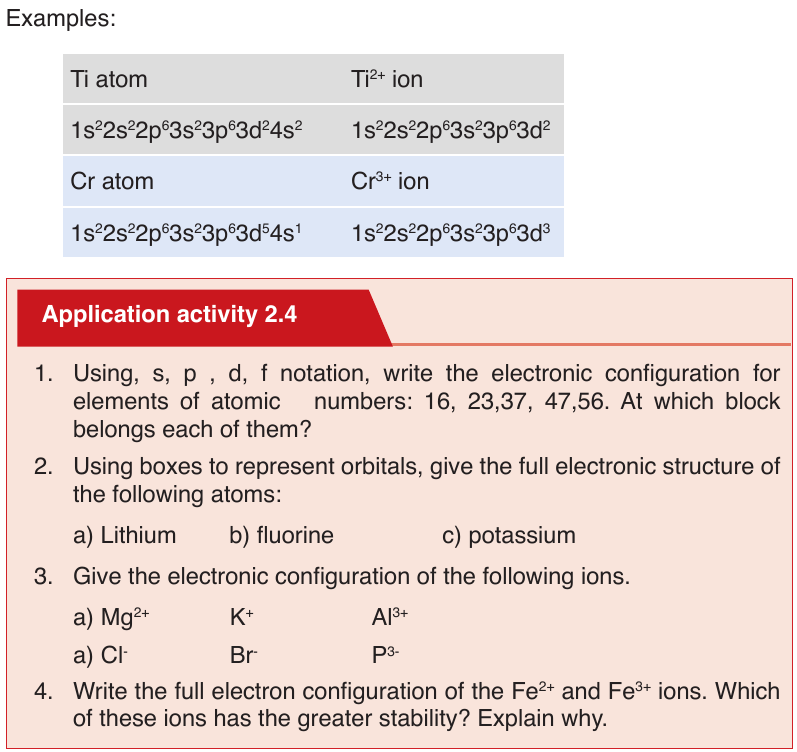
End unit assessment
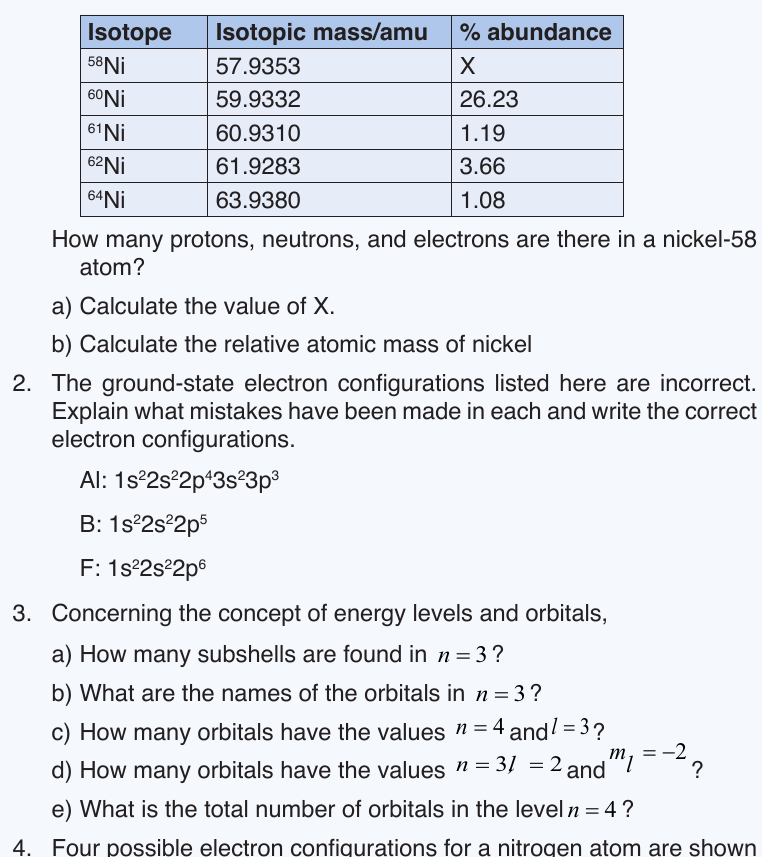
1. Given the following data concerning isotopes of nickel:

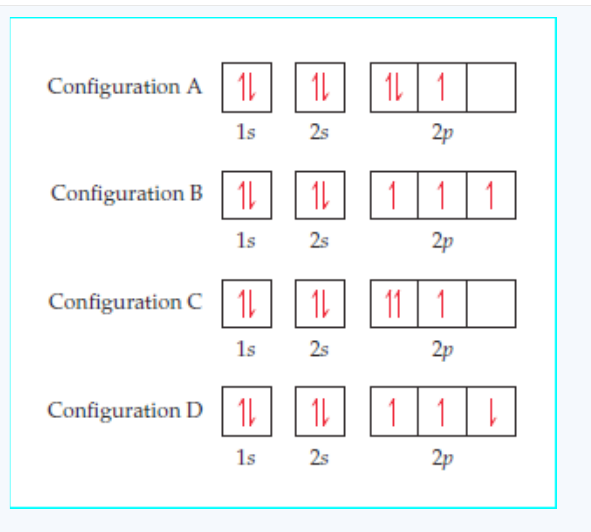
UNIT 3: CELL STRUCTURE

The three basic, structural parts of a compound microscope are: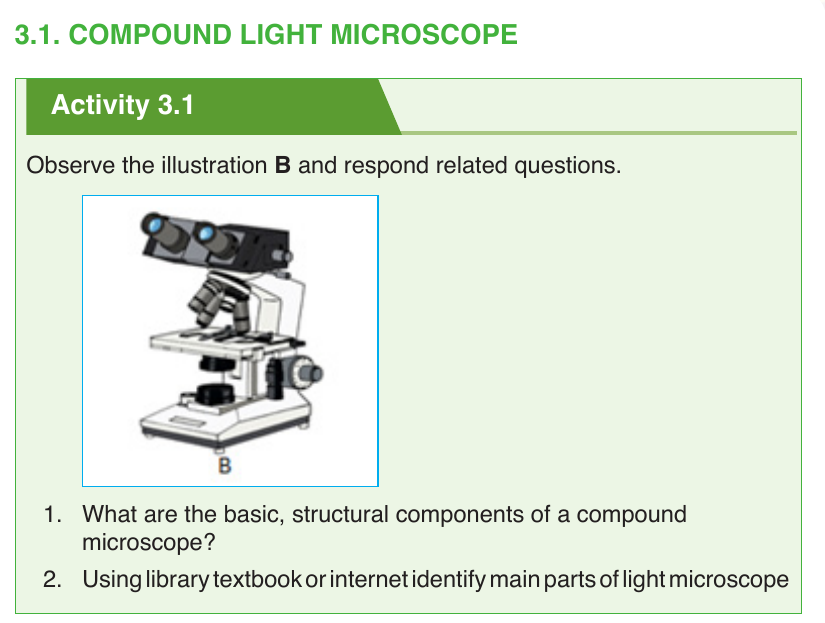
–– head/body houses the optical parts in the upper part of the microscope,
–– base of the microscope supports the microscope and houses the illuminator;
–– arm connects to the base and supports the microscope head
The different parts of light microscope are described below:
–– Base: supports and stabilizes the microscope on the table or any other working place
–– Light source: It is made by lamp or mirror which provides light for viewing the slide.
–– Stage: is a platform used to hold the specimen in position during observation.
–– Stage clips: are pliers used to fix and hold tightly the slide on stage.
–– Arm: supports the body tube of microscope
–– Body tube: maintains the proper distance between the objective and ocular lenses
–– Arm: used for holding when carrying the microscope and it holds the body tube which bears the lenses.
–– Coarse focus adjustment moves stage up and down a large amount for coarse focus
–– Fine focus adjustment moves stage up and down a tiny amount for fine focus
–– Objective lenses: focuses and magnifies light coming through the slide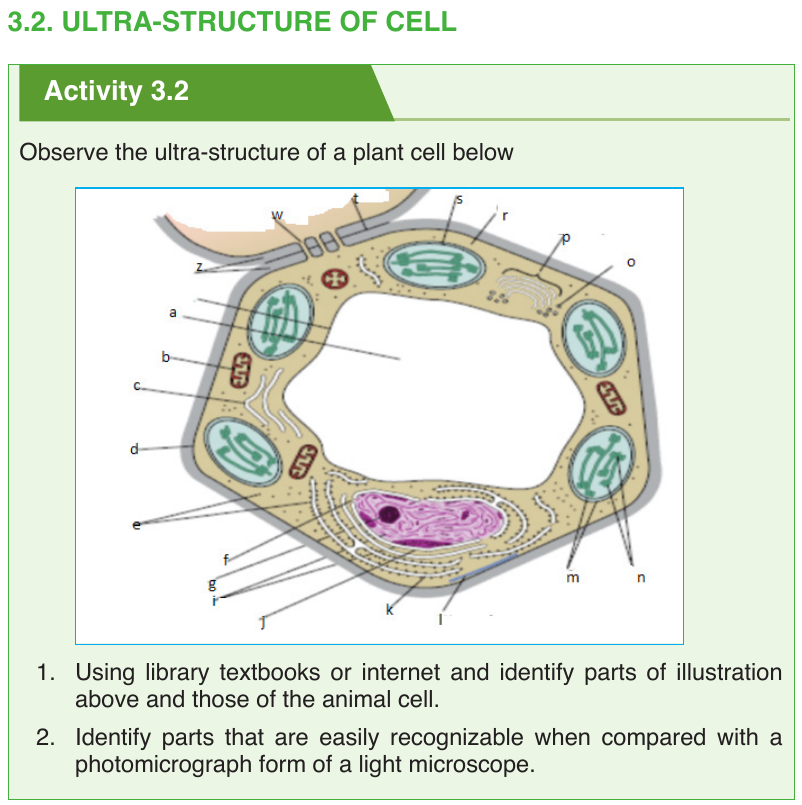
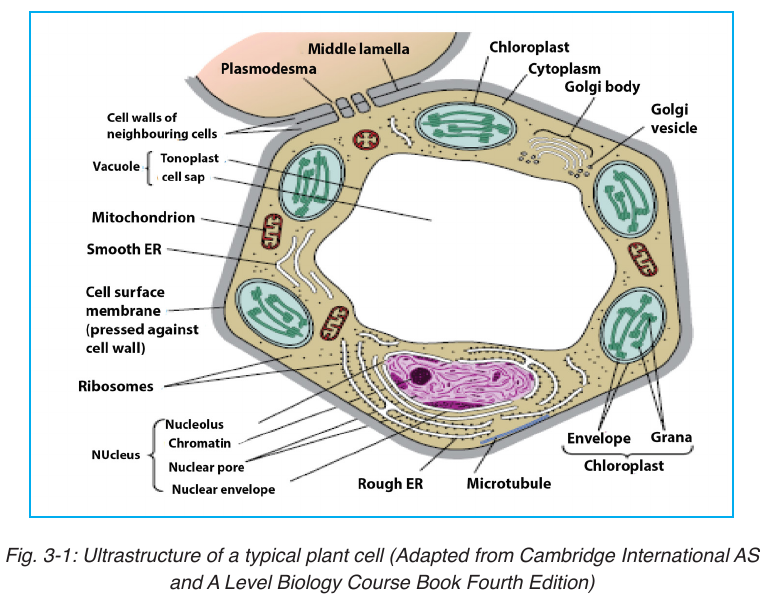
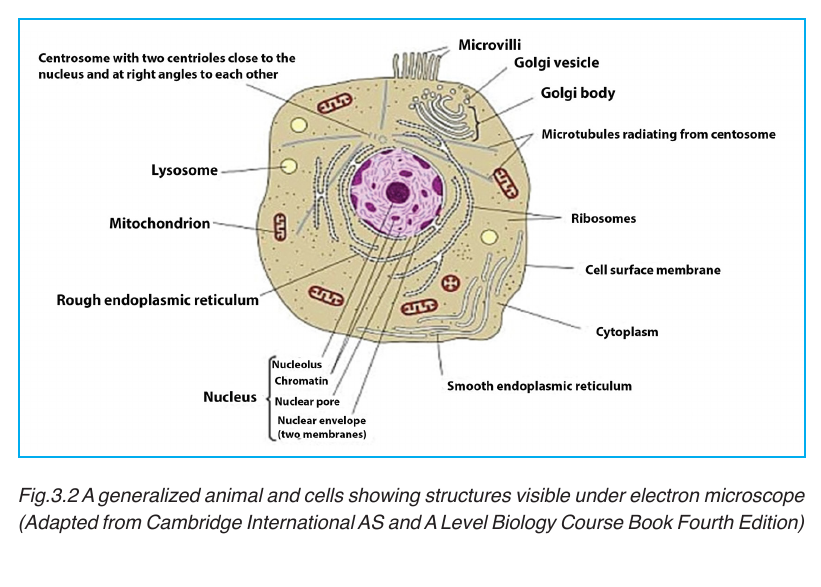
Under electron microscope, it is possible to identify a range of organelles in
plant and animal cells. Ultrastructure is the detailed of cell as revealed bythe electron microscope.
–– Gives the membranes of some eukaryotic cells the mechanical stability.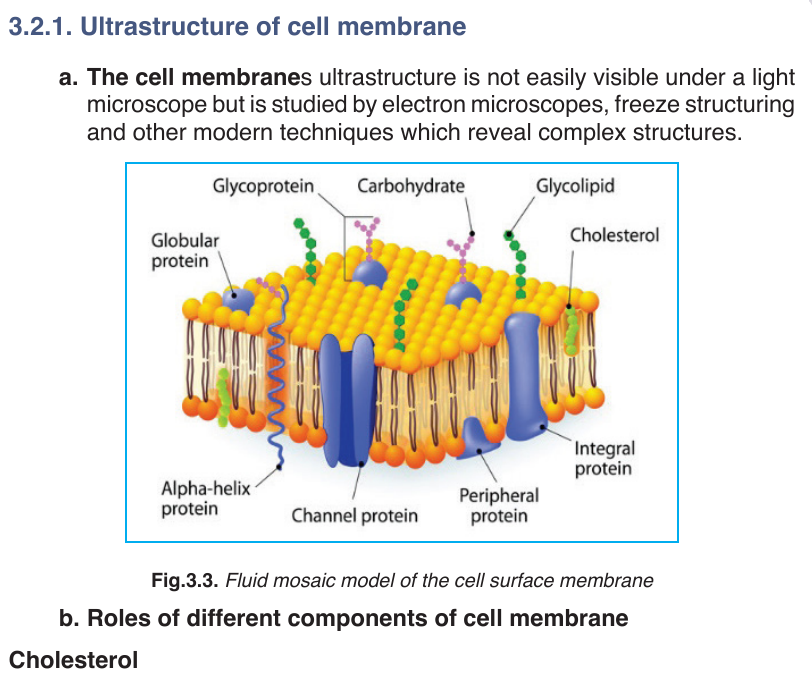
–– It fits between fatty acid tails and helps make the barrier more complete,
so substances like water molecules and ions cannot pass easily and directly through the membrane.
Channel proteins
–– Allow the movement of some substances across the membrane.
–– Large molecules like glucose enter and leave the cell using these protein channels.
Carrier proteins
–– Actively move some substances across the cell membrane. For
example, magnesium and other mineral ions are actively pumped into
the root’s hair cells from the surrounding soil.
–– Nitrate ions are actively transported into xylem vessels of plants. Receptor sites
–– Allow hormones to bind with the cell so that a cell response can be carried out.
–– Glycoproteins and glycolipids may be involved in cells signalling and they allow the immune system to recognize foreign objects to the cells.
–– Some hormone receptors are glycoprotein, and some are glycolipid.
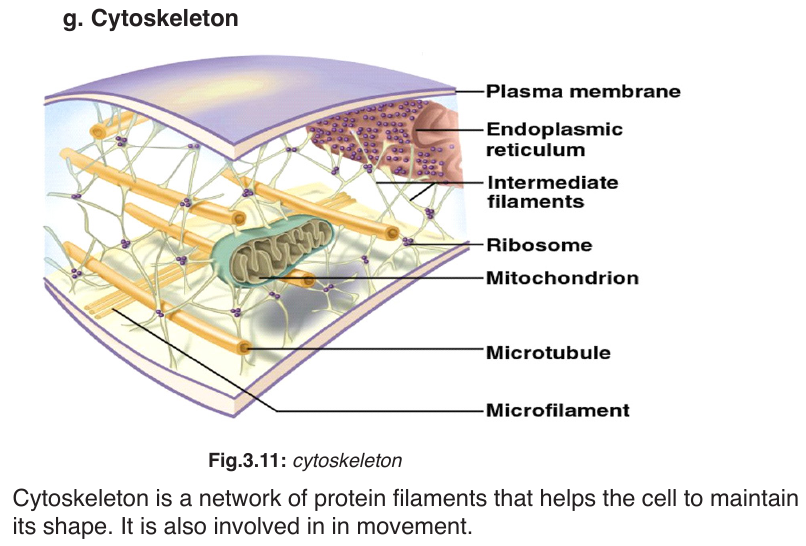
3.2.2. Cytoplasmic constituents and their functions
plant and animal cells contain a variety of cell organelles including nucleus,
mitochondria, Golgi apparatus, endoplasmic reticulum, ribosomes, centrioles,vacuoles, chloroplasts, lysosomes and cytoskeleton.
The ER consists of a series of flattened membrane-bound sacs called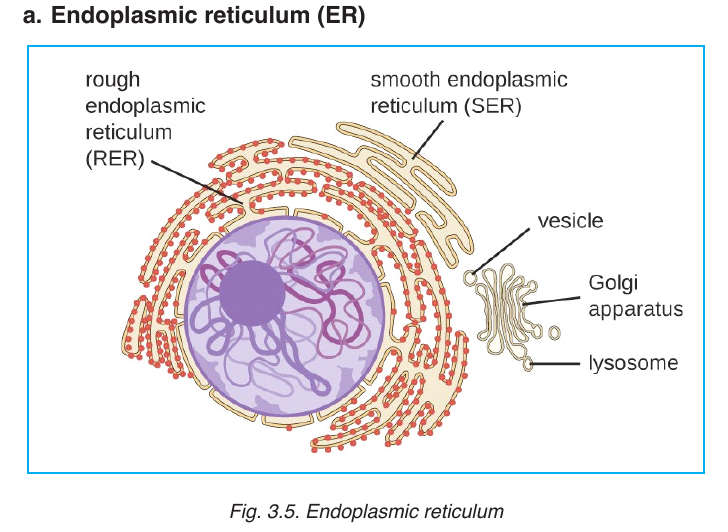
cisternae. The rough ER is surrounded with ribosomes. The rough ER
transports proteins made on attached ribosomes. The smooth ER is made
of tubular cavities lacks ribosomes, and it involves in synthesis of lipids that
the cell needs.
NB: Glandular cells are seen to have several RER for synthesis of hormones
and enzymes. Examples include liver cells, plasma cells, and pancreaticcells.
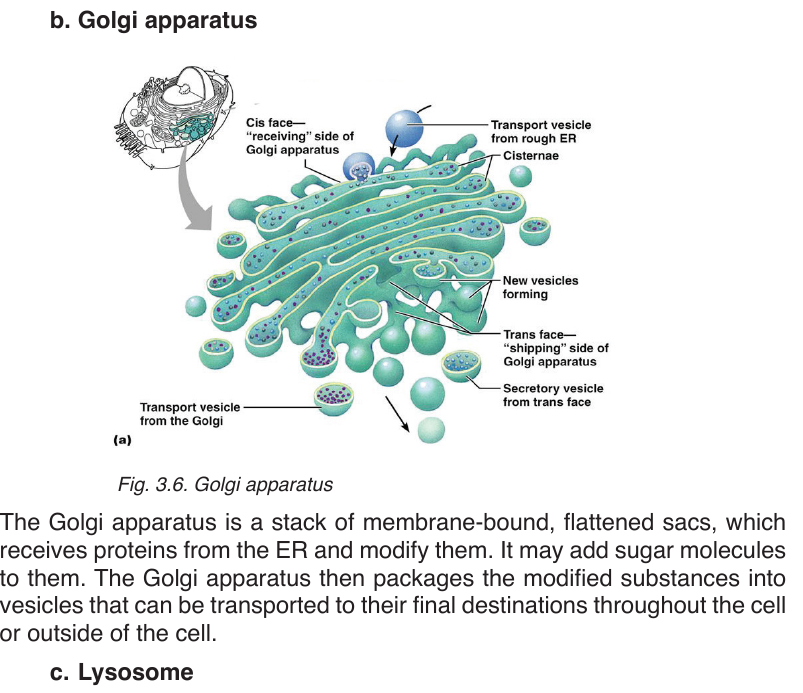
These are spherical sacs surrounded by a single membrane. They contain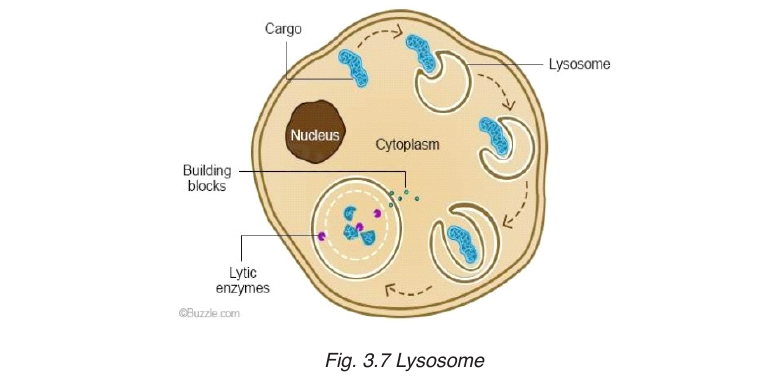
powerful digestive enzymes. Their role is to break down materials such
as white blood cells, and destroy invalid microorganisms. In acrosome,
lysosomes help the sperm to penetrate the egg by breaking down thematerial surrounding the egg.
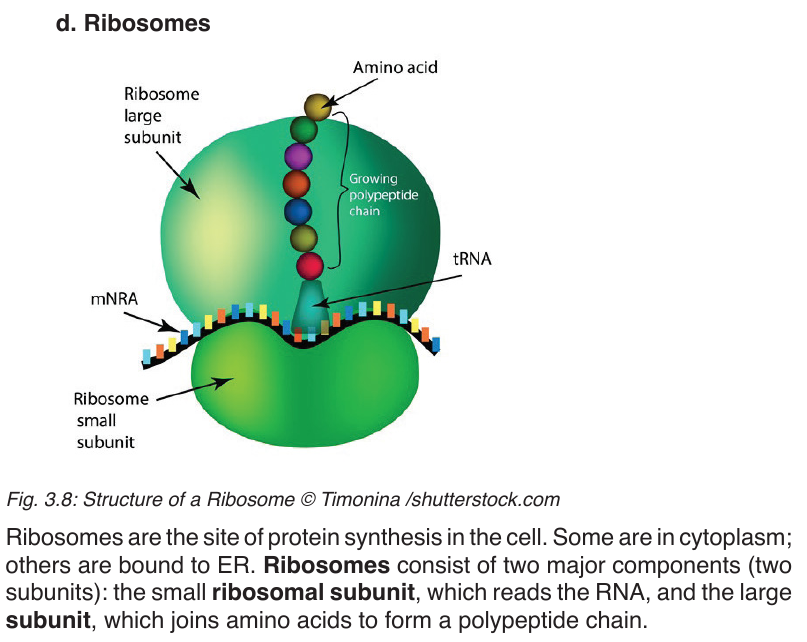
A vacuole is a saclike structure that is used to store materials such as water,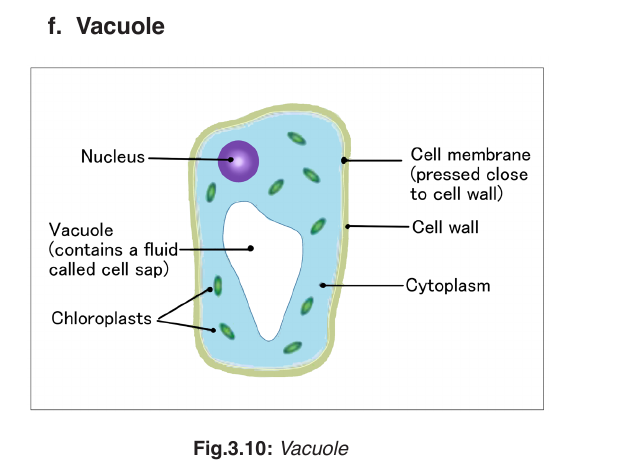
salts, proteins, and carbohydrates. In many plant cells there is a single, and
large central vacuole filled with liquid. The pressure of central vacuole in
this cells makes it possible for plants to support heavy structures such as
leaves and flowers. Some animals and some unicellular organisms contain
contractile vacuoles which contract rhythmically to pump excess water outof the cell.
The cell nucleus contains nearly all the cell’s DNA with the coded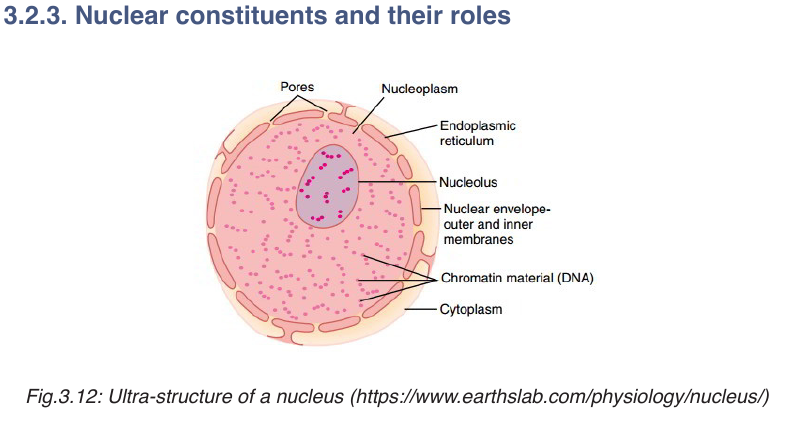
instructions for making proteins and other important molecules. The nucleus
is surrounded by a double nuclear envelope, which allow materials to move
into and out of the nucleus through nuclear pores. The granules found in the
nucleus are called chromatin which consist of DNA bound to protein. When
a cell divides, the chromatin condenses into chromosomes containing the
genetic information. The nucleus contains a dense spherical structure callednucleolus in which assembly of ribosomes occurs.
Chloroplasts are the site of photosynthesis in plant cells. These are found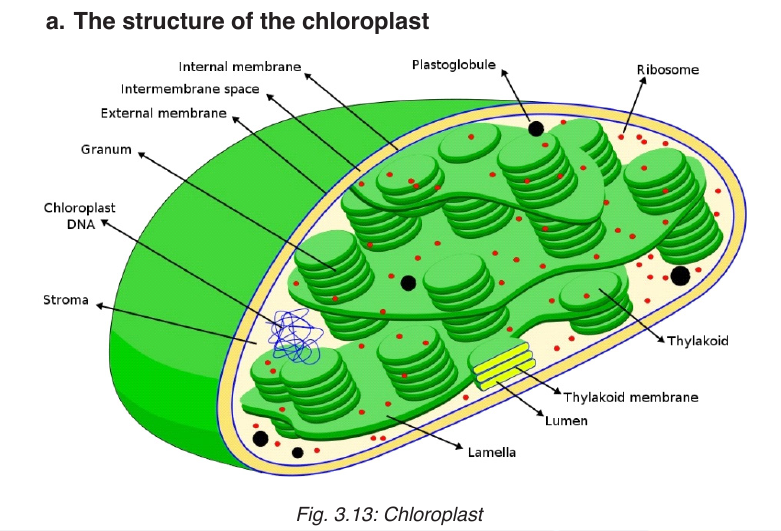
in plant cells and in cells of some protoctists. They also have two membranes
separated by a fluid-filled space. The inner membrane is continuous, with
thylakoids. A stalk of thylakoids is called a granum (plural: grana). A
chloroplast contains many sets of disc like sacs called thylakoids, which
are arranged in stacks known as grana. Each granum looks like a stack of
coins where each coin being a thylakoid. The thylakoid contains chlorophyll
molecules which capture the light energy that is needed for in the process of
light-dependent reactions of photosynthesis. A typical chloroplast contains
approximatively 60 grana, each consisting of about 50 thylakoids. The space
outside the thylakoid membranes are made by watery matrix called stroma.
The stroma contains enzymes responsible for light-independent reactions ofphotosynthesis.
Mitochondrion have two membranes separated by a fluid-filled intermembrane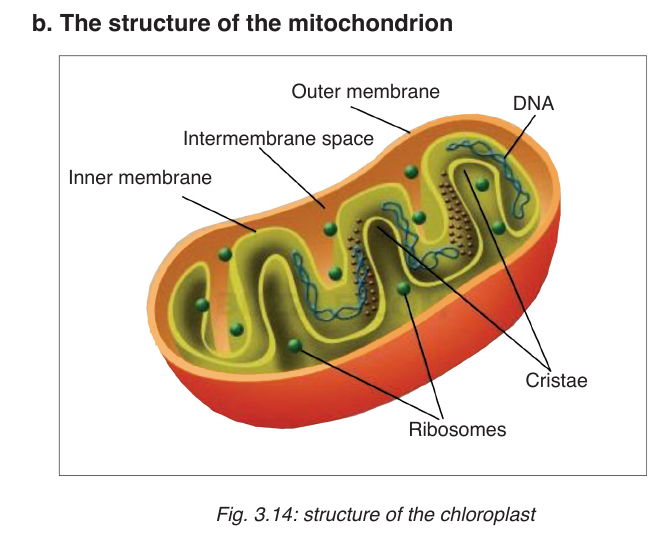
space. The inner membrane is highly folded to form cristae that plays a big
role in aerobic respiration. The central part of the mitochondrion is called
matrix. The mitochondria are the site where Adenosine triphosphate(ATP= cellular energy) is produced during aerobic respiration.
3.3.1. Similarities between animal cell and plant cell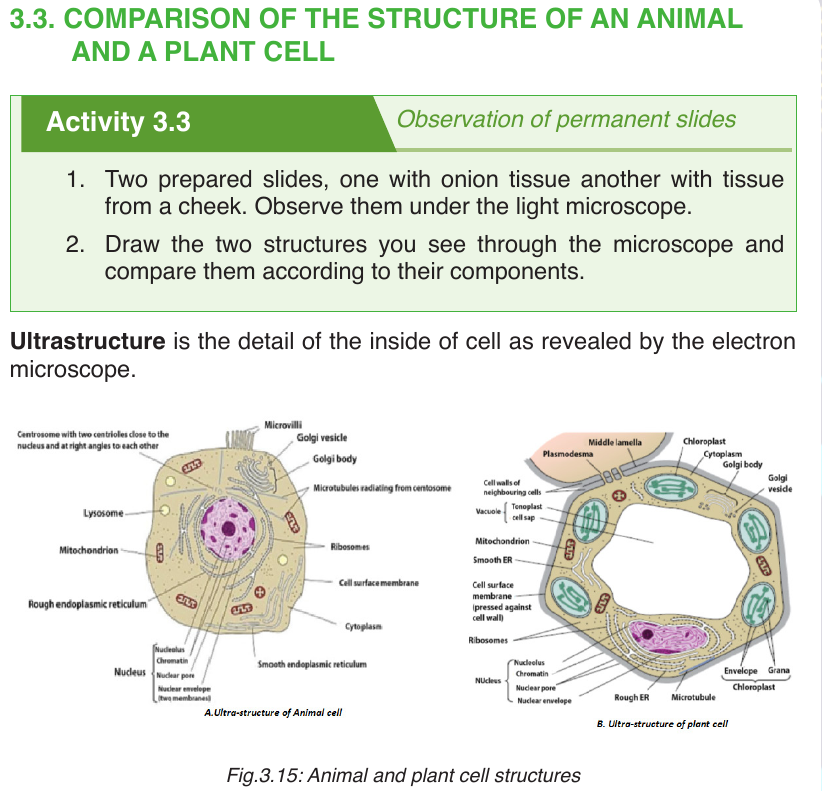
–– Both animal and plant cells have a cell membrane, a cytoplasm and a nucleus.
–– Both animal and plant cells have a true nucleus bounded by an envelope.
–– Both animal and plant cells have mitochondria, Golgi apparatus, Reticulum endoplasmic, lysosome, big ribosomes (80S), peroxisome, microtubules.
–– The protoplasm is enveloped by a bounding cell membrane called plasmalemma.
–– The protoplasm is composed of a dense round structure called nucleus which is surrounded by a less dense jelly-like cytoplasm.
–– Vacuoles contain secretions, food- particles, or decomposing organic substances.
–– Chemically, both plant and animal cells are made up of water (80-90%), proteins (7-13%), lipids (1-2%), carbohydrates (1-1.5%) and inorganic salts.
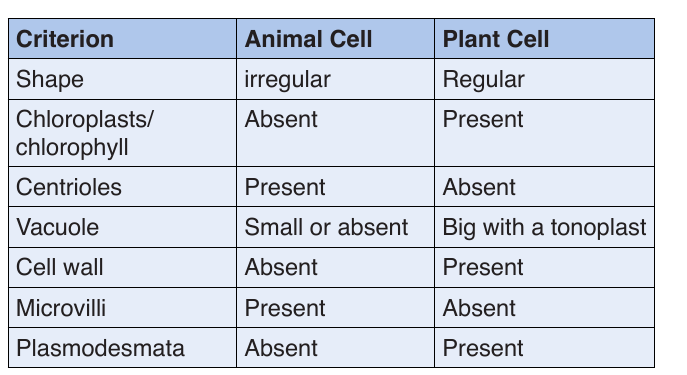
–– The cytoplasmic organelles are suspended in a semi-fluid jelly matrix called cytosol.3.3.2. Difference between animal and plant cells
End unit assessment
A. Multiple choice questions
1. Which organelle converts the chemical energy in food into a form
that cells can use?
a) Chromosome
b) Chloroplast
c) Nucleus
d) Mitochondrion
2. The cell membranes are constructed mainly of:
a) Carbohydrate gates
b) Protein pumps
c) Lipid bilayer
d) Free-moving proteins
3. In many cells, the structure that controls the cell’s activities is the:
a) Nucleus
b) Nucleolus
c) Cell membrane
d) Organelle
4. Despite differences in size and shape, all cells have cytoplasm and a
5.a) Cell wall
b) Cell membrane
c) Mitochondria
d) Nucleus
If a cell of an organism contains a nucleus, the organism is a (an)
a) Plant
b) Eukaryote
c) Animald) Prokaryote
6.Match each part of the cell (left column) to corresponding statement(right column):

7. How does a cell membrane differ from a cell wall?
8. Name the structures that animal and plant cells have in common,
those found in only plant cells, and those found only in animal cells.
9. List:
a) Three organelles each lacking a boundary membrane
b) Three organelles each bounded by a single membrane
c) Three organelles each bounded by two membranes (an envelope
10. The diagram below shows the structure of a liver cell as seen usingan electron microscope.
a) Name the parts labelled A, B, C and D.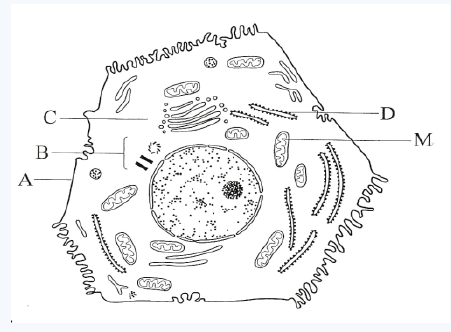
b) The magnification of the diagram above is 12 000. Calculate the
actual length of the mitochondrion labelled M, giving your answer in μm. Show your working.
c) Explain the advantage to have a division of labor between different cells in the body.UNIT 4: INTRODUCTION TO BIODIVERSITY

Species is a group of closely related organisms which are capable of
interbreeding to produce fertile offspring. Occasionally two organisms which
are genetically closely related but not of the same species can interbreed
to produce infertile offspring. For example, a cross between a donkey and
a horse, produces a mule, which is infertile. Hence, a donkey and a horse
do not belong in the same species. Another example includes lions and
tigers belonging in different species. However, when a male tiger mates
with a female lion they can have fertile offspring called tiglon, although the
offspring of female tigers and male lions called ligers are not fertile. Note
that normally tigers are forest dwellers and lions are plains dwellers and they
are ecologically isolated. Breeding has only been observed in captivity.
An ecological population is a group of individuals of the same species
which live in a particular area at any given time.
An ecological community consists of populations of different species
which live in the same place at the same time, and interact with each other.
A habitat is a specific area or place in which an individual organism lives.
When a habitat is very small it is regarded as a microhabitat.
Within the habitat, an ecological niche is the status or the role of an
organism in its habitat or the mode of life of an organism within its habitats.
For example, insects are pollinating agents and preys of insectivores.
In an environment, communities are influenced either by abiotic components,
also called abiotic factors. These are the non-living physical aspects of the
environment such as the sunlight, soil, temperature, wind, water, and air.
Communities are also influenced by biotic components, or biotic factors.
These are the living organisms in the environment.
The biosphere is the whole of the earth’s surface, the sea and the air that is
inhabited by living organisms. The biosphere is made up of all ecosystems.
An ecosystem is a collection of all the organisms that live together in aparticular place, together with their nonliving, or physical environment.
Biodiversity is defined as the full range of variety and variability within and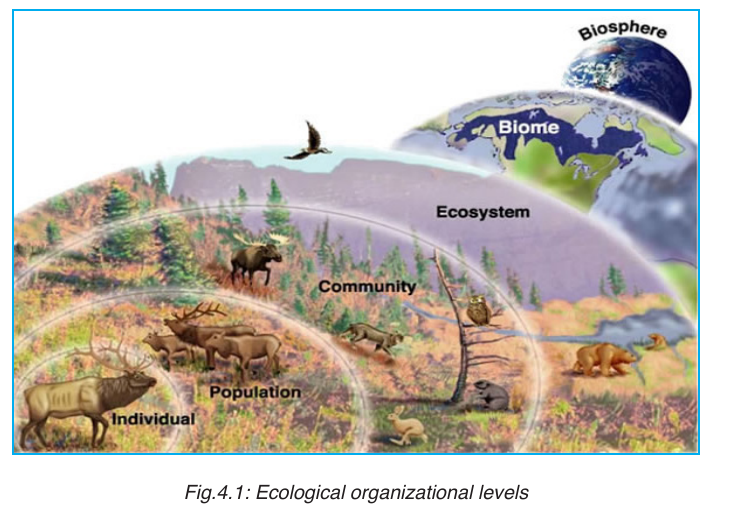
among living organisms and the ecological complexes in which they occur.
In other words, biodiversity is the variety of life. It refers to the totality of the
species including the genetic variation represented in the species populations,
across the full range of terrestrial organisms, including vertebrates andinvertebrates, protista, bacteria and plants.
Biodiversity is can be categorized into three groups: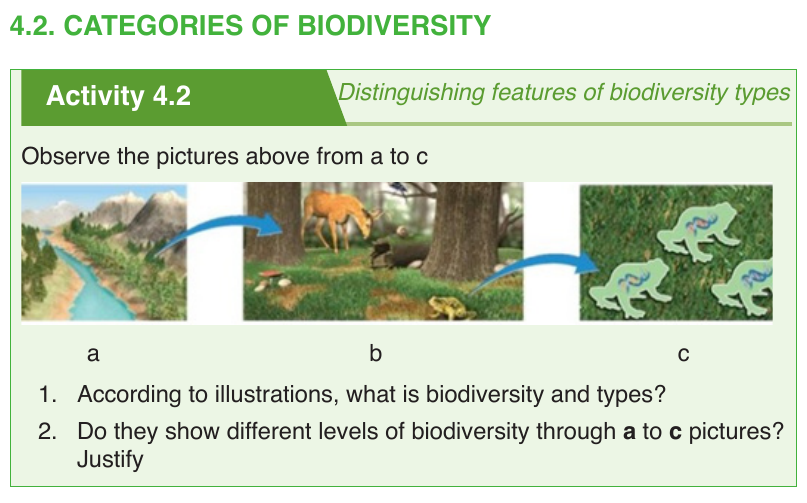
–– Genetic diversity (c): The combination of different genes found within
a population of a single species, and the patterns of variation found
within different populations of the same species. These variations are
caused by the gene mutations or chromosomal mutations which create
differences in individuals of the same species.
–– Species diversity (b): This is concerned with variation in number of
species and their relative abundance in an area in which they inhabit.
All species are different from each other. These could be structural
differences, such as the difference between a mango tree and a cow.
They could also be functional differences, such as the differences
between bacteria that cause decay and those that help us to digest
food. The variation in the relative abundance of species within a habitat
may be caused by different factors, mainly environmental factors which
can affect their rate of reproduction.
–– Ecosystem diversity (a) : This is concerned with variations in
ecosystems or habitats that occur within a region. Environmental
factors like climate change may cause diversity of habitats or systems
within a region.
–– Functional diversity
Biodiversity / biological diversity means the variability among living
organism from all sources and ecological complex of which they
are part. In general a species rich ecosystem is presumed to have
high functional diversity, because there are many species with manydifferent behaviour.
Looking anywhere around we can help us appreciate the beauty biodiversity
gives our environment. Beyond beauty, why is biodiversity important?
Biodiversity and its maintenance are very important for sustaining life on
earth. The points below guide carrying out of importance of biodiversity:
1. Importance to the nature
Biodiversity maintains food chain in the nature, all living things in environment
are interdependent. Animals could not exist without green plants. These
plants could not exist without animals to pollinate them. These plant are
dependent on decomposers. Whereas some living things can be niches for
others living things. Thus, living things have many complex relationships
among organisms.
They are adapted to live together in communities. If a species is lost from an
ecosystem the lost may have consequences for others living things in the
area. An organism suffers when a plant or animal it feed upon is removedpermanently from a food web.
Looking anywhere around we can help us appreciate the beauty biodiversity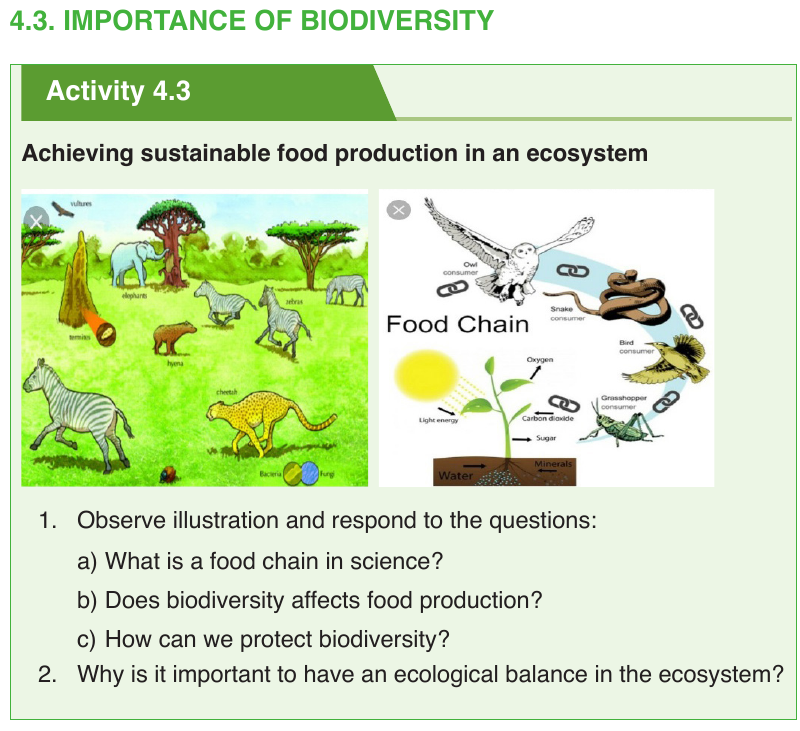
gives our environment. Beyond beauty, why is biodiversity important?
Biodiversity and its maintenance are very important for sustaining life on
earth. The points below guide carrying out of importance of biodiversity:
1. Importance to the nature
Biodiversity maintains food chain in the nature, all living things in environment
are interdependent. Animals could not exist without green plants. These
plants could not exist without animals to pollinate them. These plant are
dependent on decomposers. Whereas some living things can be niches for
others living things. Thus, living things have many complex relationships
among organisms.
They are adapted to live together in communities. If a species is lost from an
ecosystem the lost may have consequences for others living things in the
area. An organism suffers when a plant or animal it feed upon is removed
permanently from a food web.
Genetic biodiversity, arboreal plants, such as trees, tend to have more
genetic diversity, on the whole, than vascular plants, such as grasses. This
holds true both within populations and within the different species.
Large populations are more likely to maintain genetic material and thus
generally have higher genetic diversity. Hence, genetic diversity plays an
important role in the survival and adaptability of a species.
Maintaining balance of the ecosystem, a population may soon exceed the
area’s carrying capacity if its predator is removed; if the symbiotic relationship
among organisms are broken due to the loss of species, the remaining
species will also be affected.
Biodiversity protects water resources, natural vegetation cover in water
catchments help to maintain hydrological cycles, regulating and stabilising
water runoff, and acting as a buffer against extreme events such as flood
and drought.
Biodiversity increases ecosystem productivity where each species, no matter
how small, all have an important role to play, a large number of plant species
means a greater variety of crops. Greater species diversity ensures natural
sustainability for all life forms.
Promote soils formation and protection, the well-being of all plants and land-
based animals depends on the complex processes that take place in soil.
Soil develop from parent material by various weathering processes. Organic
matter accumulation, decomposition, and humification are as critically
important to soil formation as weathering.
Provision of biological resources, biodiversity provides main ecosystem
service such as nutrient cycling, carbon sequestration, pest regulation and
pollination, sustain agriculture productivity. Promoting the healthy functioning
of ecosystems ensures the resilience of agriculture as it intensifies to meet
growing demands for food production.
2. Importance to people
By diverse species of plants and algae living in variety of ecosystem through
photosynthesis process, regularly supply oxygen for breathing process to
human being. Yet only a few species of plants and animals supply the major
portion of food eaten by the human population.
Drugs companies manufacture synthetic drugs are first isolated from living
things. Example, mold penicillium provides an antibiotic penicillium, cinchona
tree release antimalarial drug etc Preserving biodiversity ensures there willbe a supply of living things, some of which may provide future drugs.
Biodiversity and food security, the provisioning of clean water and diverse
food supply makes it vital for all living things, biodiversity helps regulate the
nutrients cycle and water and mitigates impacts of climate change
3. Biodiversity stability
Biodiversity can bring stability to ecosystems. These are stable if their
biodiversity is maintained. Instead of being clumped together, the plants are
scattered in many parts of the rain forest, making it more difficult for thedisease organism to spread.
4.4.1. Threats of biodiversity
the main causes of biodiversity loss can be attributed to the influence of
human activities on ecosystems. Threats to biodiversity may include:
a. Habitat loss and the degradation of the environment
The habitat loss and the degradation of the environment occur in different
ways.
The most occurring, are tree cutting, agriculture and fires. These human
activities lead to the alteration and loss of suitable habitats for biodiversity.
As a consequence, there is a loss of plant species as well as the decrease
in the animal species associated to this plant diversity.
b. ntroduction of invasive species and genetically modified
organisms
Species originating from a particular area are harmful to native species
also called endemic species when they are introduced into new natural
environments. They can lead to different forms of imbalance in the ecological
equilibrium, so that endemic species may fail to compete with introduced
species, and they may affect the abundance and distribution in natural
habitat.
c. Pollution
Human activities such as excessive use of fertilizers, and increased pollutants
from industries and domestic sewage affect biodiversity. They contribute to
the alteration of the flow of energy, chemicals and physical constituents of the
environment and hence species may die as a result of toxic accumulation.
d. Overexploitation of natural resources
Increased hunting, fishing, and farming in particular areas lead to the decrease
and loss of biodiversity due to excessive and continuous harvesting without
leaving enough time for the organisms to reproduce and stabilize in their
natural habitat.
e. Climate change
This is a change in the pattern of weather, related changes in oceans, land
surfaces and ice sheets due to global warming resulting from man’s activities.
Increasing global temperatures have resulted into melting of icebergs raising
sea levels and so flooding coastal areas eventually affecting the niche, andthese may take the lives on many living things.
4.4.2. Consequences of loss of biodiversity
They are various consequences of loss of biodiversity that include:
–– Desertification, is thought by scientists to be a consequence of climate
change, has been considered to be related to deforestation. Disrupting
water cycles and soil structure results into less rainfall in an area.
–– Floods as a result of rising sea levels.
–– Habitat destruction for extensive farming, timber harvesting and infrastructure and settlement.
–– Decrease in food production as result of change in pattern of weather that affects productivity
–– Large scale deforestation has a negative effect on nutrient recycling and can accelerates soil erosion.–– Diseases that come as effects of floods and malnutrition due to famine
1.Explain what is meant by a habitat and make a list of all the habitats you can see in your school compound.
2. Pollution is one of the causes of aquatic biodiversity loss.
a) What do you understand by water pollution?
b) Outline human activities that contribute to water pollution
c) Discuss how polluted water affects aquatic living organisms?d) Relate desertification with biodiversity loss.
UNIT 5: INTRODUCTION TO CLASSIFICATION
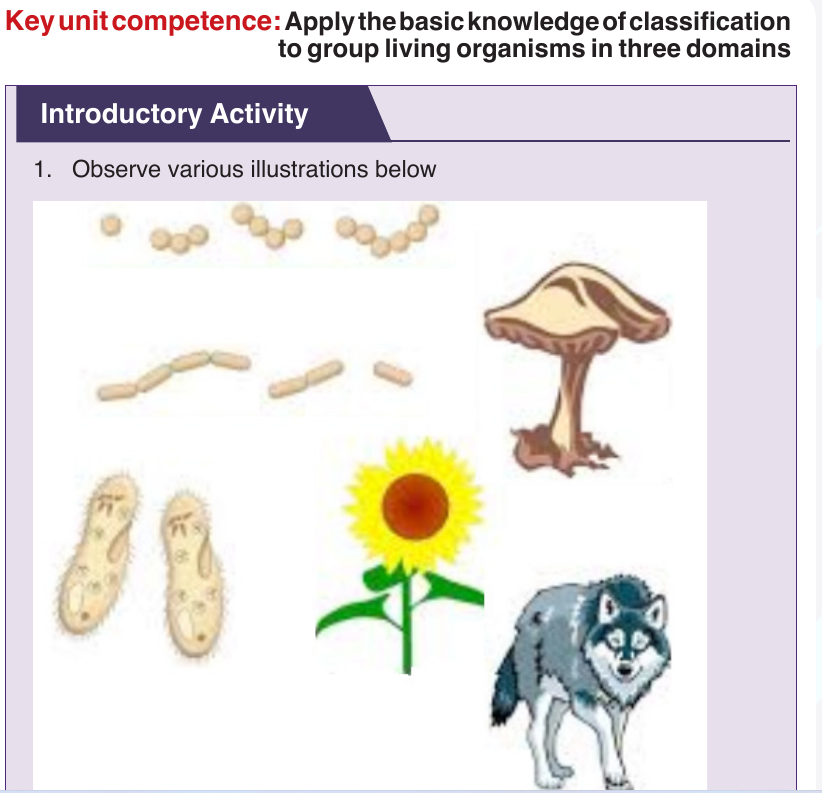

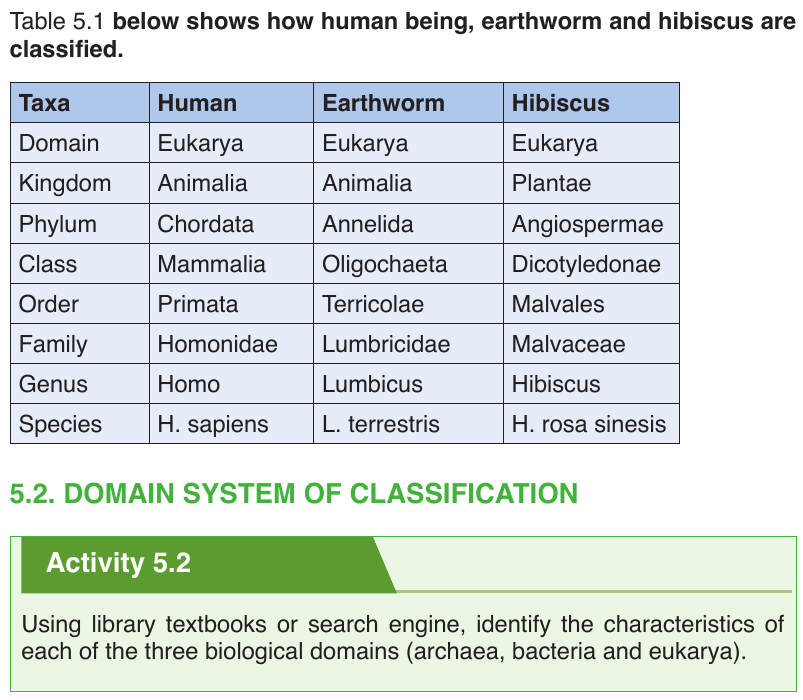
Taxonomy is the study of classification of living organisms in taxonomic
levels called taxa (singular: taxon). In biological classification, these taxa
form a hierarchy. Each kind of organism is assigned to its own species, and
similar species are grouped into a genus (plural: genera). Similar genera are
grouped into a family, families into an order, orders into a class, classes into a
phylum (plural: phyla) and phyla into a kingdom. The hierarchy classification
starts from the largest group, the domain.
The eight levels of classification are known as taxa (taxon in singular),
these include: Domain, Kingdom, phylum, class, order, family, genus
and species. As one moves down the taxonomic hierarchy, it follows that
the number of individuals decreases but the number of common featuresincreases.
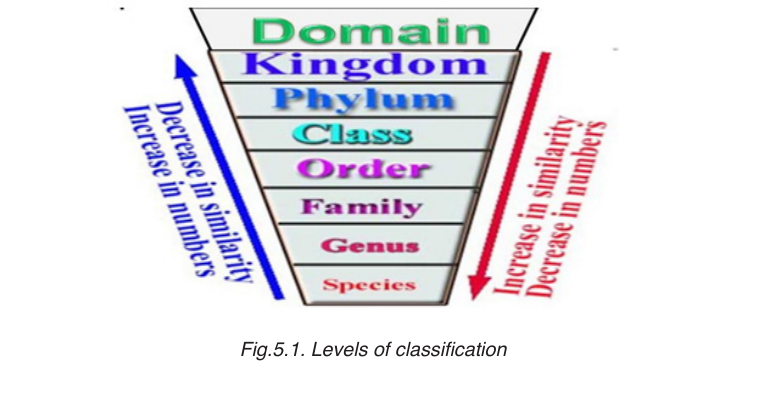
 Three domains are used by biologists to divide organisms into three large
Three domains are used by biologists to divide organisms into three large
groups based on their cell structure. The domain is the highest taxon in the
hierarchy. The prokaryotes are divided between the domains Eubacteria/
Bacteria and Archaebacterial/ Archea, while all the eukaryotes are placed
into the domain Eukarya.
5.2.1. Domain eubacteria/ bacteria
domain bacteria include prokaryotic organisms as their cells do not have
defined, membrane-limited nuclei.
They are all microscopic that vary in size between 0.2 to 10 micrometers.
The characteristic features of bacteria are:
–– Cells with no true nucleus
–– DNA exists in circular chromosome and does not have histone proteins associated with it.
–– No membrane-bound organelles (mitochondria, endoplasmic reticulum, Golgi body, chloroplasts)
–– Contain mesosomes as infolding of membrane and acts as sites for respiration as they lack mitochondria.
–– Ribosomes (70 S) are smaller than in eukaryotic cells
–– Cell wall is always present and contains peptidoglycans in place of cellulose
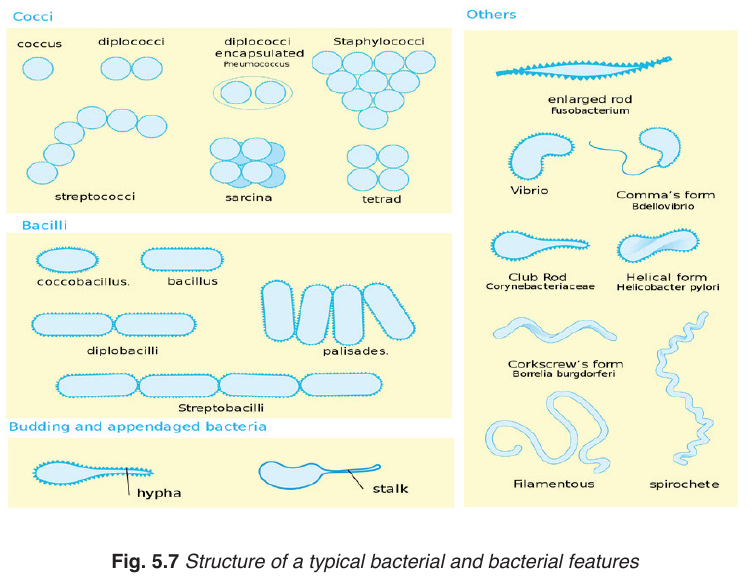
–– Cells divide by binary fission– Usually exist as single cells or colonies.
The bacteria are important when they help to fertilize fields, to recycle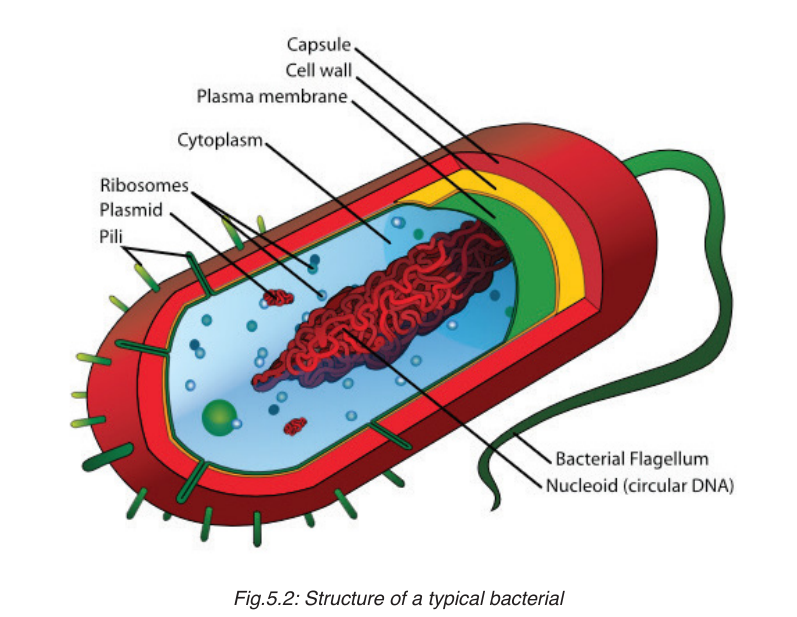
nutrients on earth, and to produce food and medicines. The bacteria that
live in soil recycle the nitrogen and carbon contained in the complex organic
molecules that remain in plants and animals after they have died. While
most bacteria is found in many disease, bacteria is very useful to our lives
because is found in the digestive system to help break down food.
a. Domain Archaea (Archaebacteria)
This contains bacteria that live in extreme environments where few other
organisms can survive, like in volcanic hot springs and black organic mud
totally devoid of Oxygen.
Types and economic importance
They are classified according to the environments they live in:
–– Methanogenic bacteria that live in habitats deprived of oxygen and give
off methane as a product of metabolism for example those that live in
the guts of ruminant animals such as cows.
–– Halophilic bacteria live only in water with high concentration of salt.
–– Thermoacidophilic bacteria tolerate extreme acid and temperature that
exceed boiling point of water and a pH below 2.They are autotrophic
producer for a unique animal community’s food chain.
b. Domain Eukarya
All the organisms classified into this domain have cells with true nuclei
and membrane-bound organelles. It include the four remaining kingdoms:
protists, fungi, plantae and Animalia. Their characteristic features are:
–– Cells with a nucleus and membrane-bounded organelles
–– linear DNA associated with histones arranged within a chromosome inthe nucleus
–– Ribosomes (80S) in the cytosol are larger than in prokaryotes, while chloroplasts and mitochondria have small ribosomes (70S ribosomes), like those in prokaryotes.
–– Chloroplast and mitochondrial DNA is circular as in prokaryote suggesting an evolutionary relationship between prokaryotes and eukaryotes
–– A great diversity of forms: unicellular, colonial and multicellular organisms
–– Cell division is by mitosis.–– Many different ways of reproduction including asexually and sexually.
5.3.1. Protoctista
This kingdom is made up of a very diverse range of eukaryotic organisms,
which includes those that are often called protozoans and algae. Living
things such as paramecium, amoeba, euglena, algae and plasmodia belongto the kingdom Protoctista.
The characteristic features of protoctists are listed according to the different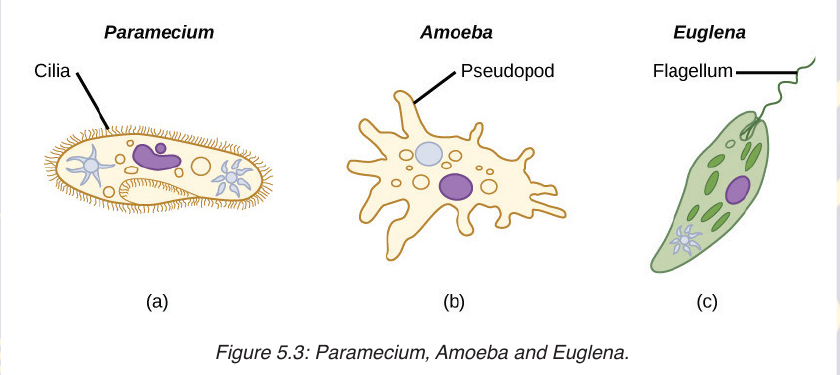
phyla due to their diverse range:
–– Rhizopus that have pseudopodia for locomotion. Example, amoeba.
–– Flagellates which are protoctista which move by using flagella. Example, Trypanosoma.
–– Sporozoans which are mainly parasitic organisms that reproduces by multiple fission. Example plasmodium.
–– Ciliates are protoctista which move with cilia. Example paramecium.
–– Euglenoid flagellates which are organisms with flagella but with a biochemistry quite distinct from that of flagellates. Example Euglena.
–– Green algae are photosynthetic protoctista with chlorophyll pigments. Example chlorella.
–– Red algae are photosynthetic protoctista with red pigment as well as chlorophyll. Example, chondrus
–– Brown algae which are photosynthetic protoctista with brown pigments as well as chlorophyll. Example Fucus and sea weed.
NB: Some protists are used in food industry. eg saccharomyces cerevisiae
( yeast). The plants protists produce almost one half of the oxygen on the
planet through photosynthesis. They participate in decomposition andrecycling of nutrients that humans need to live.
5.3.2. Fungi
Fungi are all heterotrophic, obtaining energy and carbon from dead and
decaying matter or by feeding as parasites on living organisms. There is avast range in size from the microscopic yeasts to macroscopic fungi.
–– Heterotrophic nutrition.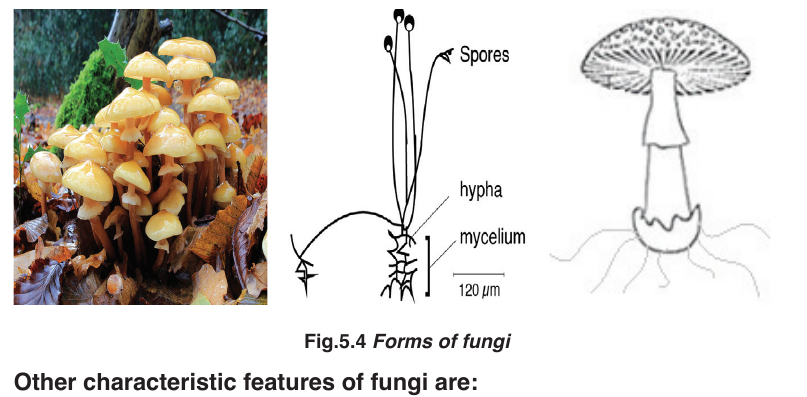
–– They use organic compounds made by other organisms as their sourceof energy and source of molecules for metabolism
–– Reproduce asexually by means of spores and sexually by conjugation.
–– Simple body form, which may be unicellular or made up of long threads called hyphae (with or without cross walls).
–– Large fungi such as mushrooms produce large compacted masses of hyphae known as fruiting bodies to release spores.
–– Cells have cell walls made of chitin or other substances.
NB: As economic importance some mushrooms are used as food,
saprophytic fungi such as mucor spp/Rhizopus spp are used in the curing
of tea and tobacco; the fungi decompose organic matter helping to clean the
environment and recycle materials.
5.3.3. Plantae
Plants are all multicellular photosynthetic organisms. They have complex
bodies that are often highly branched both above and below the ground.
Characteristic features of plants are:
–– Multicellular eukaryotes with cells that are differentiated to form tissues and organs.
–– Few specialized cells.
–– Cells have large and often permanent vacuoles for support with cell walls made of cellulose.
–– Autotrophic living organisms (most plants contain chlorophyll and store carbohydrates as starch or sucrose).
–– Usually plants are green
–– Roots ,stems and leaves
–– Sexual and asexual reproduction
NB: People depend upon plants to satisfy such basic human need as food,
clothing, shelter and health care.
5.3.4. Animalia
Animals are multicellular organisms that are all heterotrophic with differentmethods of obtaining their food.
Organisms in Animalia kingdom share the following features: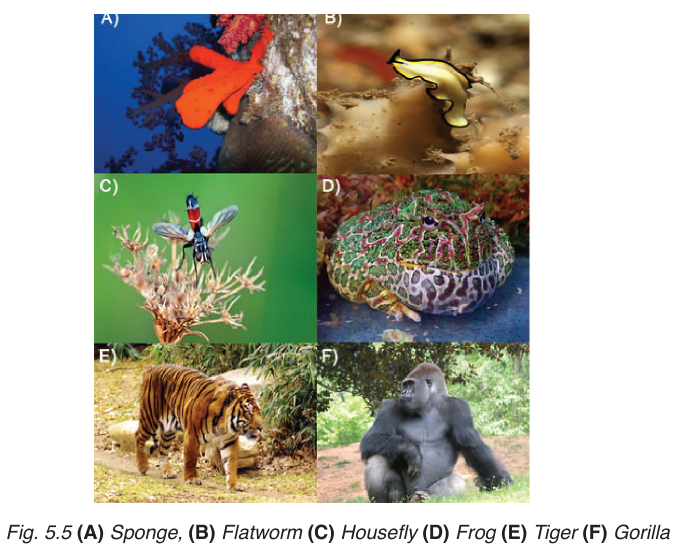
–– Multicellular (different types of specialized cells).
–– Eukaryotic
–– Heterotrophic (cells do not have chloroplasts and cannot photosynthesize,
although some, such as coral polyps have photosynthetic protoctistsliving within their tissues).
–– Cell vacuoles are small and temporary (for example lysosomes and
food vacuoles).
–– Cells do not have cell walls.
–– Sense organs (communication is by the nervous system)
–– Motile, at least for part of their life
NB: Many animals are helpful to humans; many varieties of livestock are
kept because they add protein to our diets in the form of meat, milk products,
and egg. Fiber bearing animals such as sheep provide material for making clothing
5.3.5. Monera
Organisms in this kingdom are unicellular, that do not have a nucleus. They
are prokaryotic. They are the smallest and simplest organisms. Some of
them stick together to form chains or clusters while others are single cells.
The figure below shows a typical structure of a bacterial cell which contains
all the main features of prokaryotes.
Although some of them are harmful in causing human diseases, others are
beneficial species that are essential to good health, as they are involved infood industry, medicine and in pharmacy.
NB: About economic importance of monera kingdom, many of Nostoc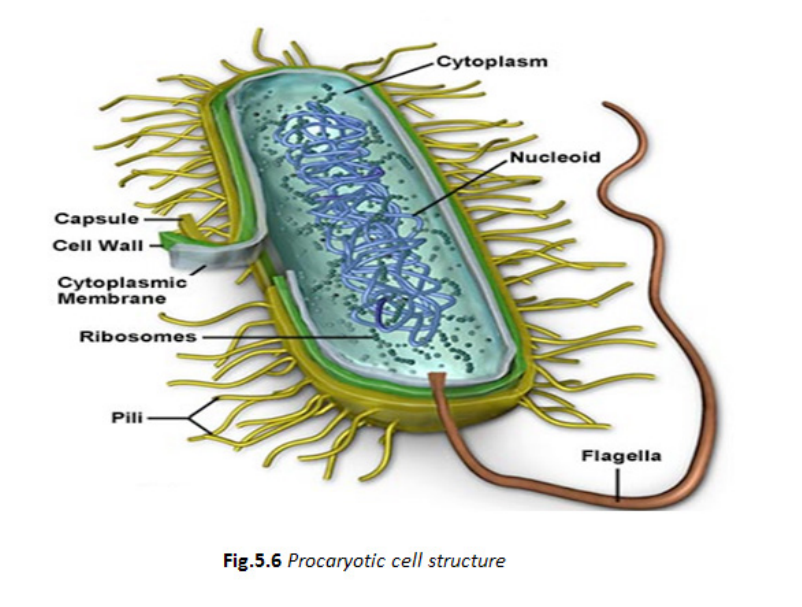
species fix atmospheric nitrogen and thus increase soil fertility. They are
also important in the manufacturing and services industries ( eg production
of many dietary supplements and pharmaceuticals)
–– Presence or absence of the envelope: Plant viruses’ bacteriophage
are no enveloped while animal viruses like HIV and influenza virus are
enveloped.
5.4.2. Characteristics of viruses
Viruses are microorganisms whose structure is only visible with electron
microscopes. A typical virus consists of DNA or RNA within a protective
protein coat called capsid which provides protection. Viruses become active
in metabolism only once inside the host cell. When they infect cells, they
use biochemical machinery and proteins of the host cell to copy their nucleic
acids and to make proteins coats often leading to destruction of the host
cells. The energy for these processes is provided by the ATP from the host
cell. Because viruses do not consist of cells, they also lack cell membranes,
cytoplasm, ribosomes, and other cell organelles. Without these structures,
they are unable to make proteins or even reproduce on their own. Instead,
they must depend on a host cell to synthesize their proteins and to make
copies of themselves. Viruses infect and live inside the cells of living
organisms. They are also regarded as parasites since they depend entirely
on living cells for their survival. Although viruses are not classified as living
things, they share two important traits with living things: They have geneticmaterial, and they can evolve.


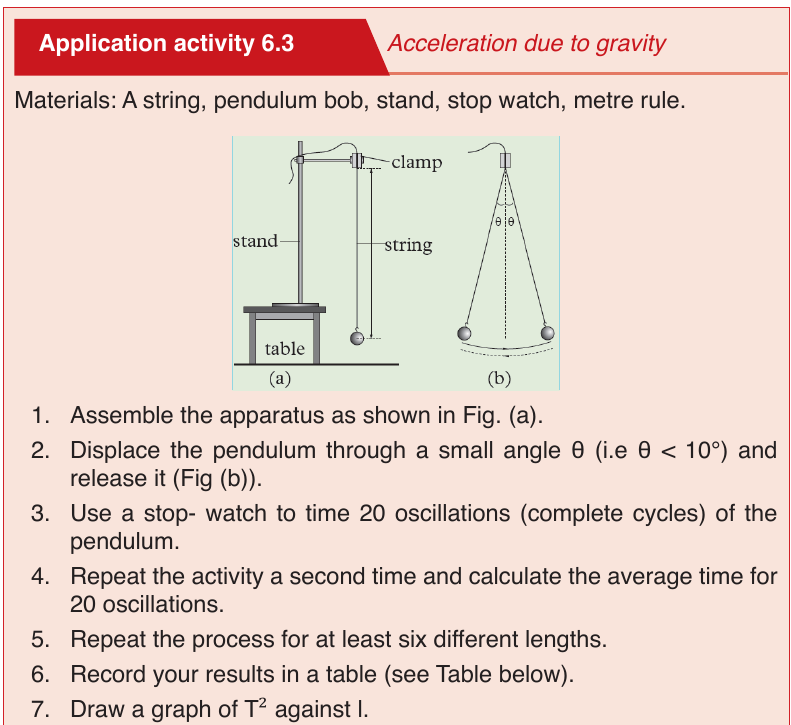
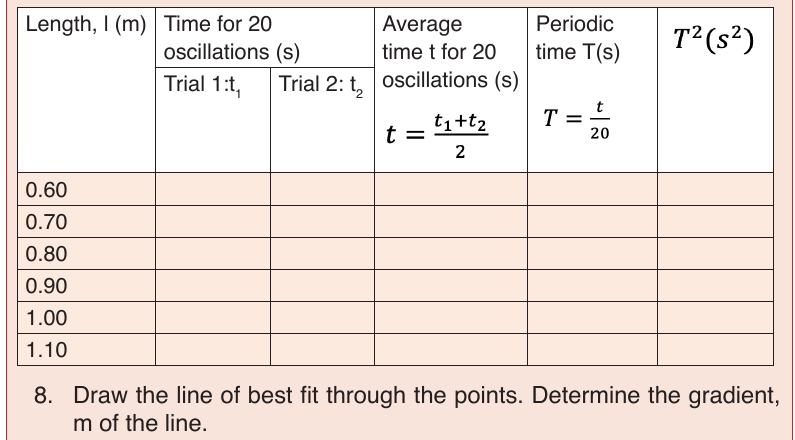
UNIT 6: MOTION ON A STRAIGHT LINE
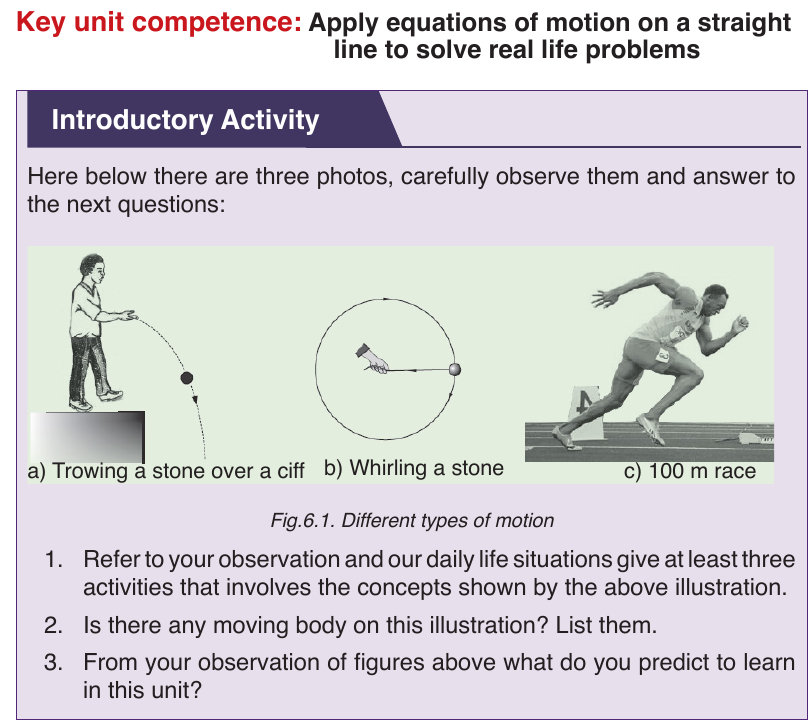
In our daily lives, we come across various objects moving from one point to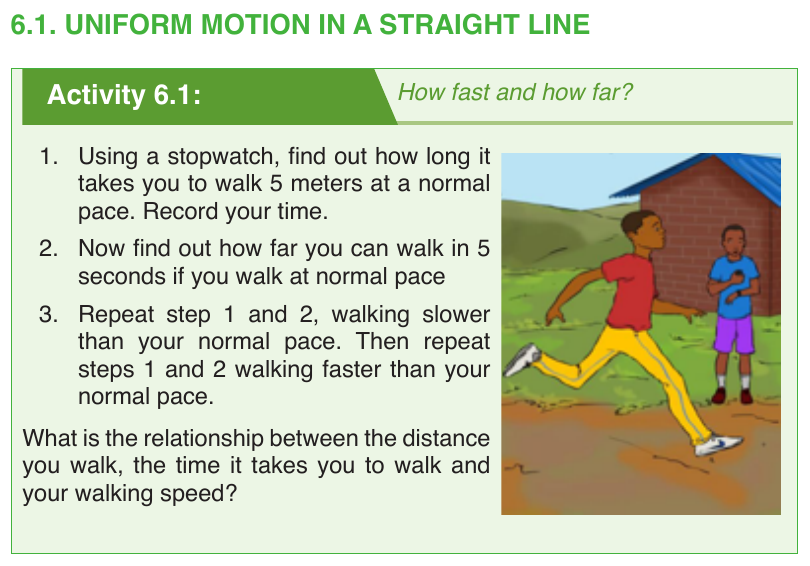
the other. The objects are said to be in motion. People, animals and machines
are from time to time involved in motion in different directions. Motion in a
straight line is called linear motion.
In this unit, we are going to study linear motion. We shall pay attention to the
time taken, distance covered, speed, velocity and acceleration of the motion
and their relationships.
There are two types of linear motion namely: uniform motion and non-uniform
motion.
Uniform motion
In this motion, the speed of the moving remains the same or constant.
Non-uniform or uniform accelerated motion
In this motion the speed of an object changes at a constant rate, a goodexample is the free fall.
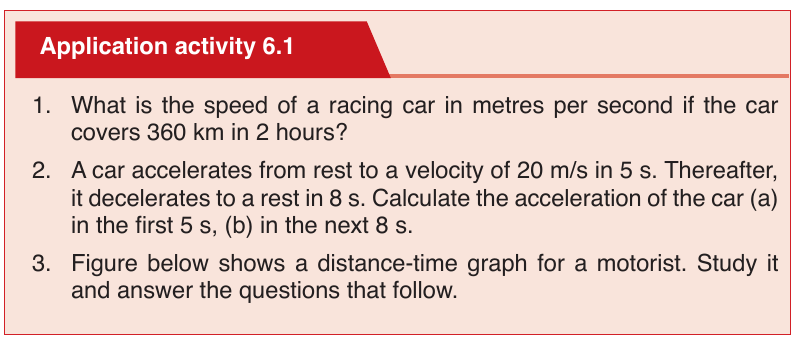
6.1.1. Distance and displacement
Distance
Distance is the total length of the path followed by an object, regardless of
the direction of motion. It is a scalar quantity and measured in units of length.
The SI unit of distance is the metre (m). Long distances may be measured in
kilometers (km) while short distances may be measured in centimeters (cm)
or millimeters (mm).
It should be noted that in determining the distance between two points, the
direction at any point along the path is not considered. The direction alongthe path may keep on changing or remain constant.
Displacement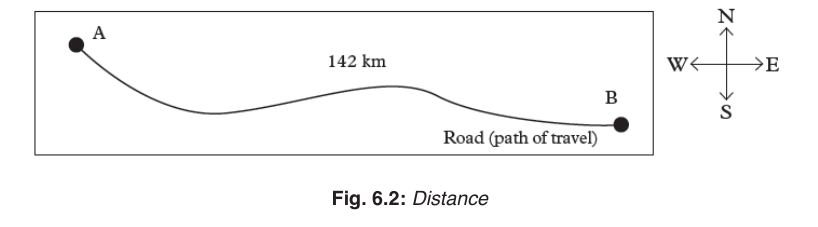
Displacement is the object’s overall change in position from the starting to
the end point. It is the shortest distance along a straight line between two
points in the direction of motion. The SI unit of displacement is the metre (m).
To fully describe displacement, you need to specify how far you have
travelled from where you started and in what direction you have travelled.
For example, point A is 100 kilometres Northwest of point B. In diagrams,
an arrowhead indicates the direction of motion (. Displacement is a vectorquantity.
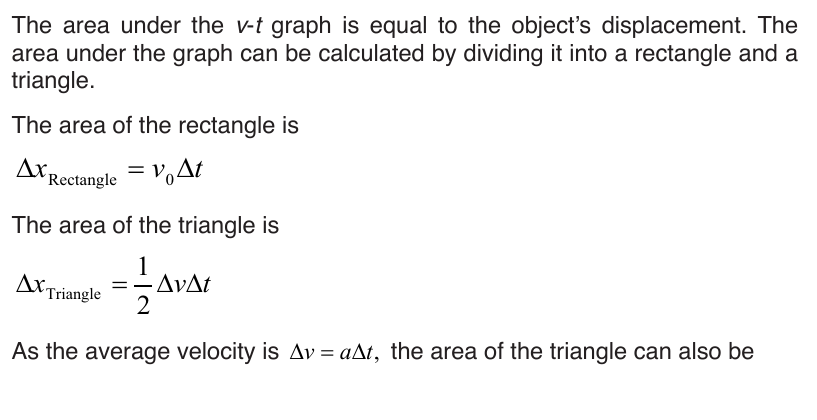
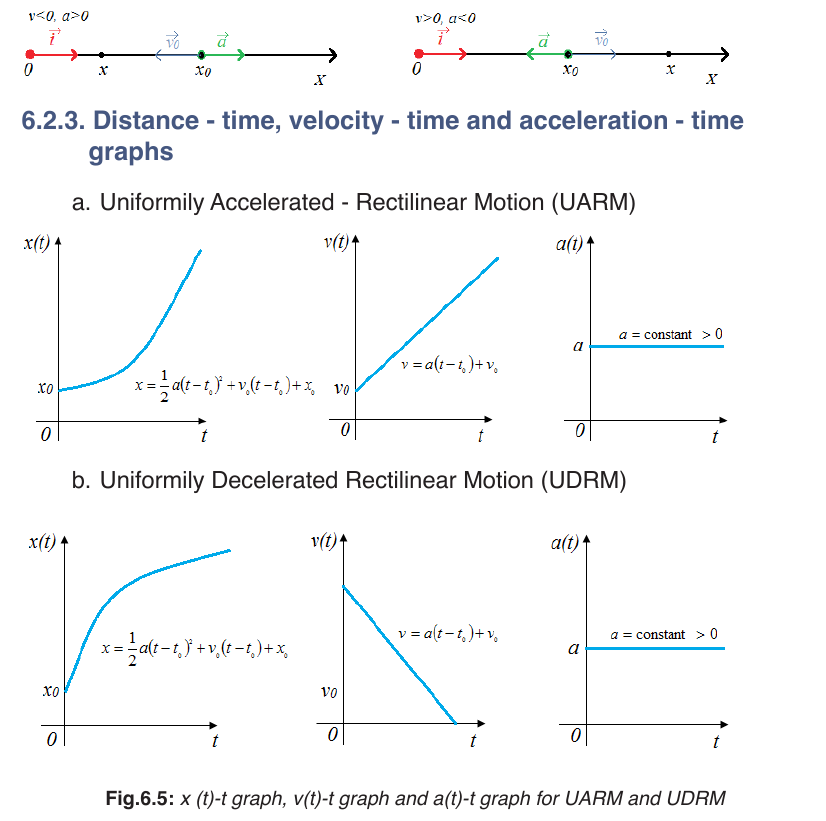
6.1.2. Average velocity and instantaneous velocity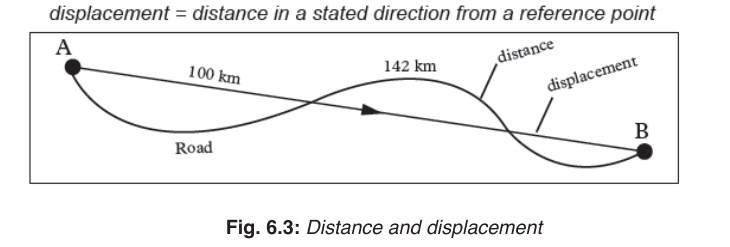
velocity or speed may be defined as the rate at which something happens,
moves or functions within a time interval, that is, how fast is a progress,
movement or an operation. In general, the average speed of an object is
defined as the covered distance divided by the time it takes to travel this
distance.
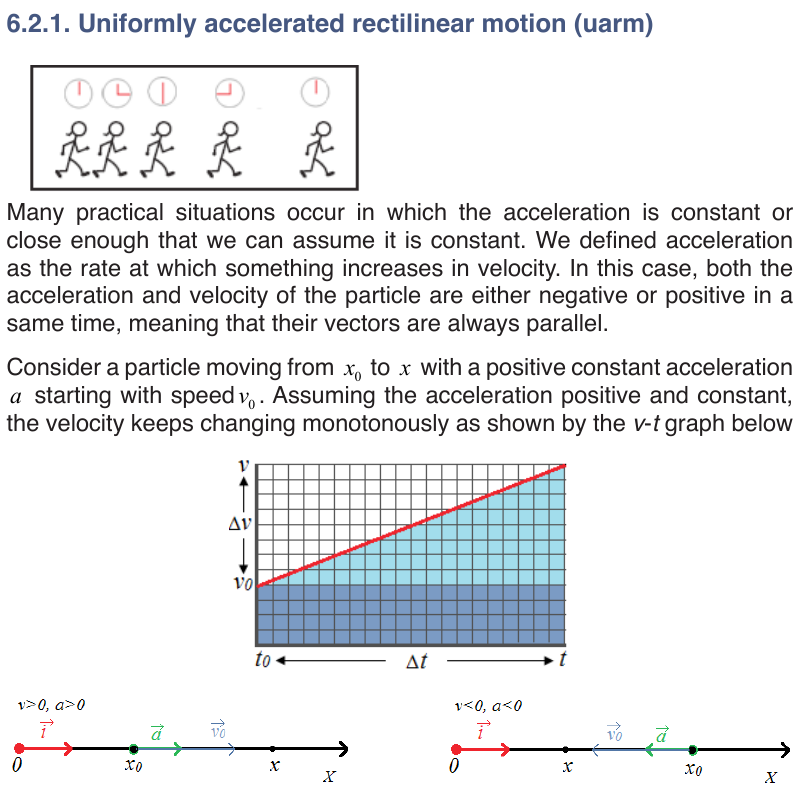
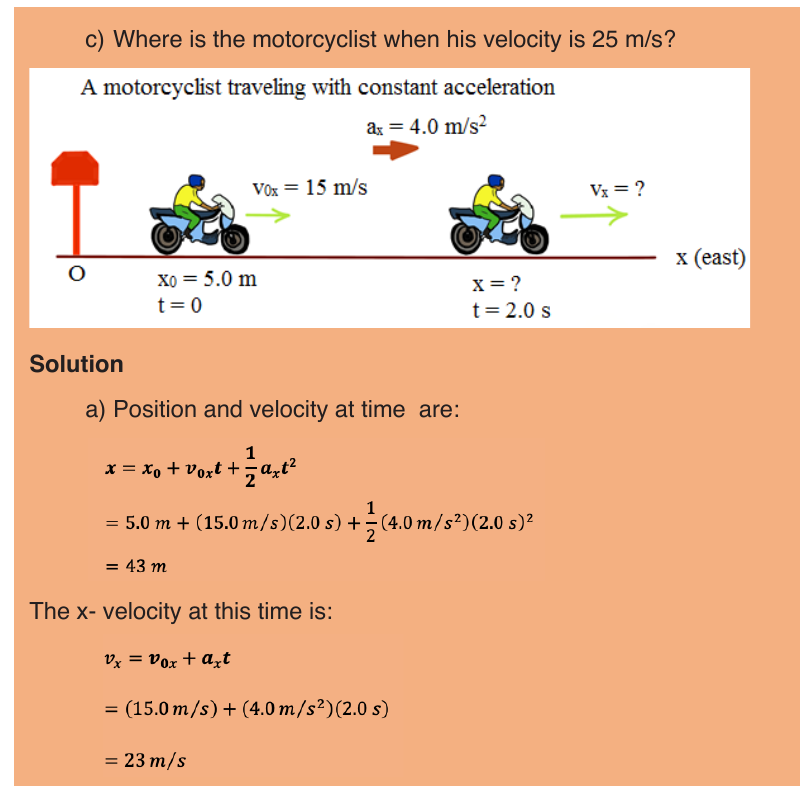
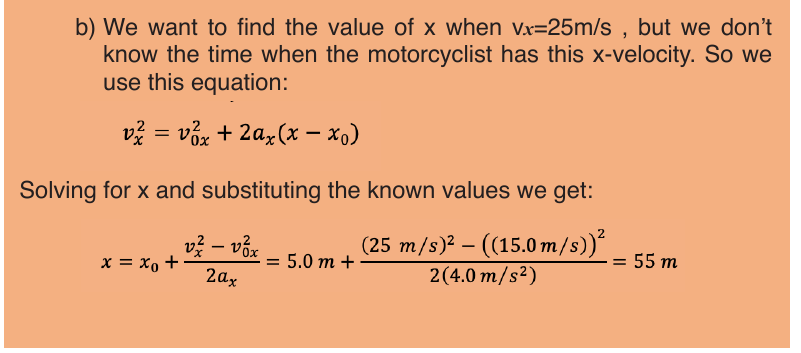
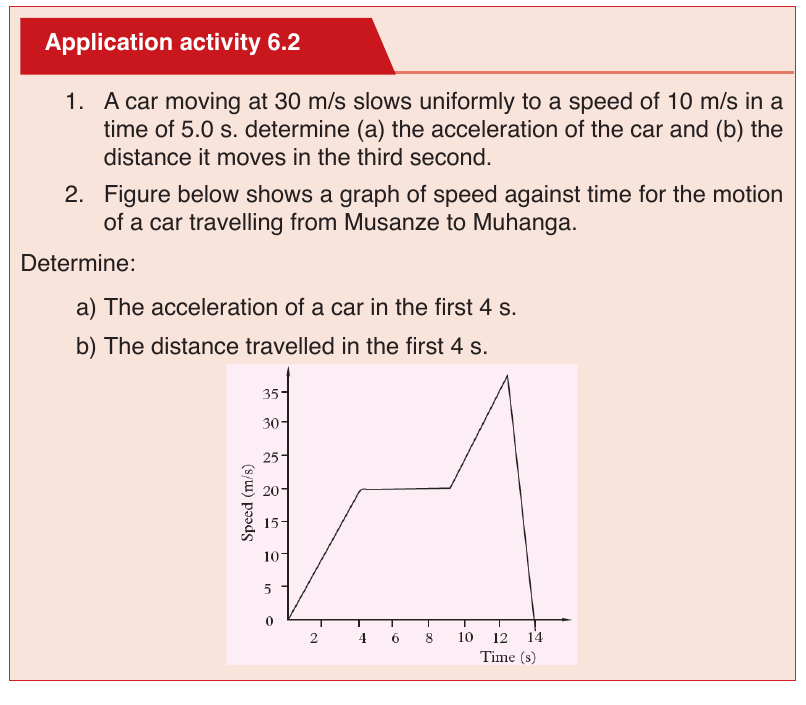
Suppose that an object is moving on X-axis so that at an instant t 0 , the
object is at point (coordinate) x0, and at another later instant t the object is
at point x.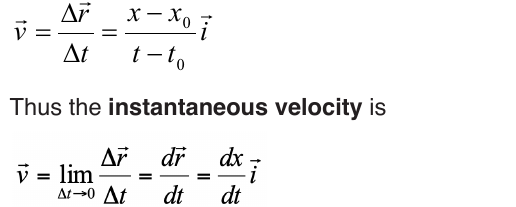
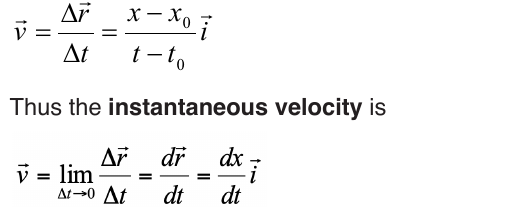
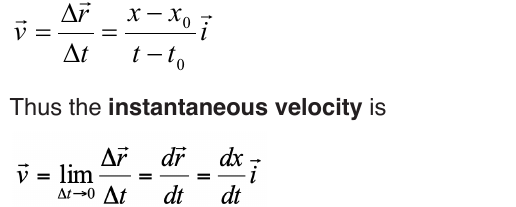



One of the most common examples of uniformly accelerated rectilinear
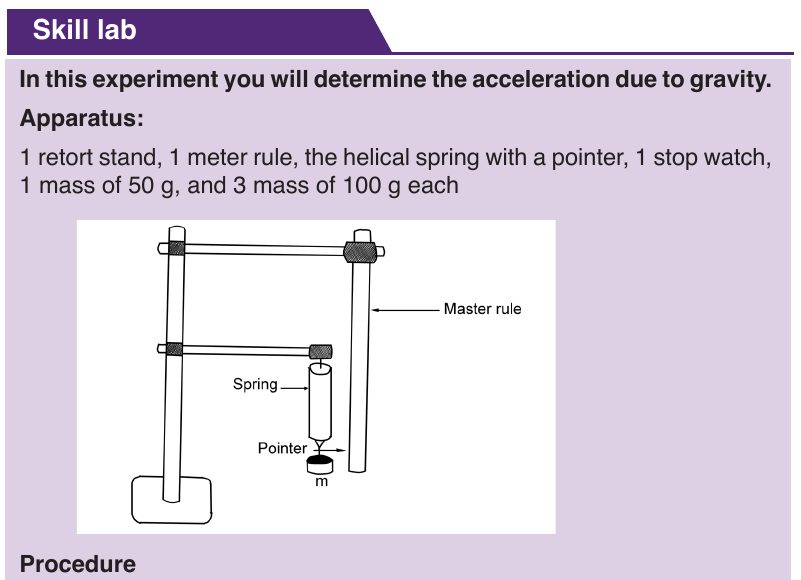
motion is that of an object allowed to fall freely near the Earth’s surface.
For free fall, Galileo postulated that: “at a given location of the Earth and in
the absence of air or other resistance all objects fall with the same constant
acceleration”. In the free-fall motion air resistance is negligible and the action
can be considered due to gravity alone.
We call this acceleration the acceleration due to gravity on the Earth, and
we give it the symbol g . Its magnitude is approximately g = − 9.8 m / s 2 . g
varies slightly according to latitude and elevation. The effects of air resistance
are often small, and we will neglect them for the most part. We will also
suppose that an object moves along Y-axis.
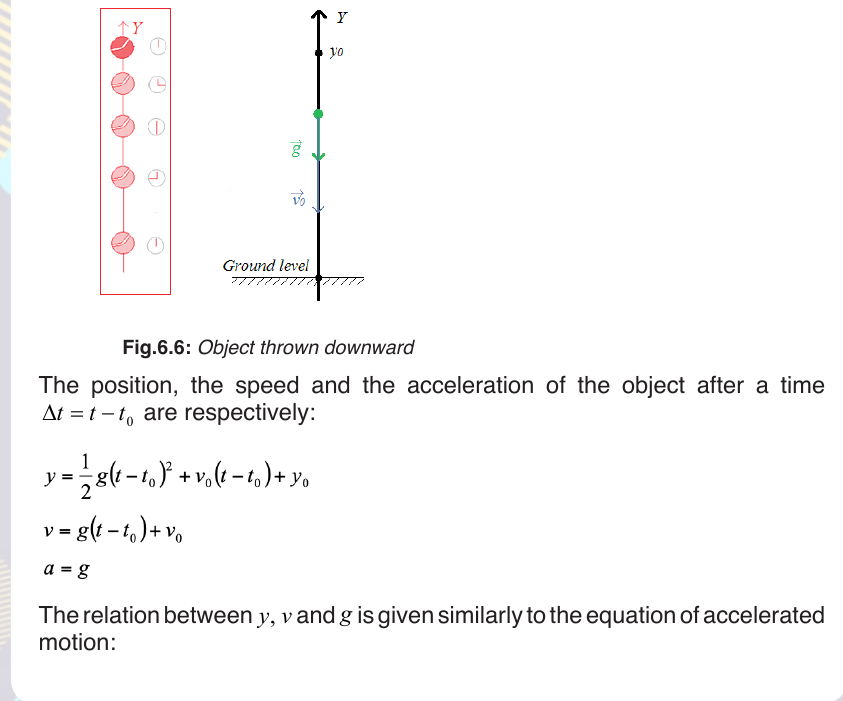
6.3.1. Object thrown downward
a. General condition
Suppose an object released to fall from a height y 0 . The vectors of g and
v0 are parallel. The following figure shows how the ball accelerates withinequivalent time intervals.