UNIT6:Natural and Industrial Processes
My goals
After studying this unit, I will be able to:
⦿ Describe a natural, biological, environmental, industrial or mining
process.
⦿ Plan, write and evaluate texts with a sequence of sentences, describing
a process.
⦿ Write a sequence of sentences describing a process, with a diagram,
paying attention to connectors of time or cause and effect.
⦿ Make notes and summary of a text.
⦿ Respect natural and industrial processes role in Rwanda’s economicdevelopment
Language use
Describing a natural process
Activity 1
Discussion and research
Look at the various natural occurrences like thunderstorms, earthquakes,
stars, clouds, rain water, and others. Have you ever wondered about the
processes these natural phenomena go through to form?
Activity 2
Look at the photograph of a cloud below. Discuss the importance ofclouds.

Activity 3
Read the passage below to find out how clouds are
formed
What are clouds?
A cloud is a large collection of very tiny droplets of water or ice crystals.The droplets are so small and light that they can float in the air.
How are clouds formed?
All air contains water, but near the ground it is usually in the form of an
invisible gas called water vapour. When warm air rises, it expands and
cools. Cool air can’t hold as much water vapour as warm air, so some
of the vapour condenses onto tiny pieces of dust that are floating in the
air and forms a tiny droplet around each dust particle. When billions of
these droplets come together they become a visible cloud.
Activity 3
Read the passage below to find out how clouds are
formed
What are clouds?
A cloud is a large collection of very tiny droplets of water or ice crystals.The droplets are so small and light that they can float in the air.
How are clouds formed?
All air contains water, but near the ground it is usually in the form of an
invisible gas called water vapour. When warm air rises, it expands and
cools. Cool air can’t hold as much water vapour as warm air, so some
of the vapour condenses onto tiny pieces of dust that are floating in the
air and forms a tiny droplet around each dust particle. When billions ofthese droplets come together they become a visible cloud
Why are clouds white?
Since light travels as waves of different lengths, each colour has its
very own unique wavelength. Clouds are white because their water
droplets or ice crystals are large enough to scatter the light of the seven
wavelengths (red, orange, yellow, green, blue, indigo and violet), whichcombine to produce white light.
Why do clouds turn grey?
Clouds are made up of tiny water droplets or ice crystals, usually a
mixture of both. The water and ice scatter all light, making clouds
appear white. If the clouds get thick enough or high enough, all the light
above does not make it through, hence the grey or dark look. Also, if
there are lots of other clouds around, their shadow can add to the greyor multicoloured grey appearance.
Why do clouds float?
A cloud is made up of liquid water droplets. A cloud forms when air is
heated by the sun. As it rises, it slowly cools until it reaches the saturation
point and water condenses, forming a cloud. As long as the cloud and theair that it is made of is warmer than the outside air around it, it floats!
How do clouds move?
Clouds move with the wind. High cirrus clouds are pushed along by
the jet stream, sometimes travelling at more than 100 miles per hour
(mph). When clouds are part of a thunderstorm they usually travel at30 to 40 mph
Why do clouds form at different heights in the atmosphere?
The characteristics of clouds are dictated by the elements available,
including the amount of water vapour, the temperatures at that height,the wind, and the interplay of other air masses.
How is fog formed?
There are many different types of fog, but fog is mostly formed when
southerly winds bring warm, moist air into a region, possibly ending a
cold outbreak. As the warm, moist air flows over much colder soil or
snow, dense fog often forms. Warm, moist air is cooled from below as
it flows over a colder surface. If the air is near saturation, moisture will
condense out of the cooled air and form fog. With light winds, the fognear the ground can become thick and reduce visibility to zero.
Activity 4
Research
Think about a natural observable feature and conduct research about
how it forms. Describe the process to your classmates.
Describing a biological process
Activity 1
Discussion
Just like any living thing needs food to survive, plants also make the food
they consume for their survival. In your Science or Biology lessons, youshould have learnt about the process by which plants make their food.
Activity 2
Research and essay writing using connectors of time and
cause and effect
Study the picture below carefully. Use it and your own Science
or Biology knowledge to write about the process by which plants
manufacture their food called photosynthesis. Write down the process
and choose a secretary who will present your essays to the class forfurther discussion and comparison of your findings.
Describing a process: Photosynthesis
Describing an environmental process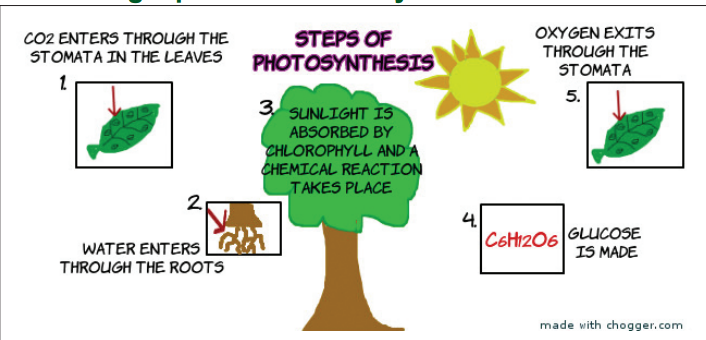
In all our studies, we have emphasised the relevance of protecting our
environment, yet human-induced factors, especially through industrialisation,continue causing insurmountable damage to the environment.
Activity 1
Discussion
(a) Have you ever heard about acid rain? What is it?
(b) What causes acid rain?
(c) Describe the effects of acid rain.
(d) What piece of advice would you suggest to prevent acid rain?
Activity 2
Study the diagram carefully. Read the passage below itto match what you see in the text.
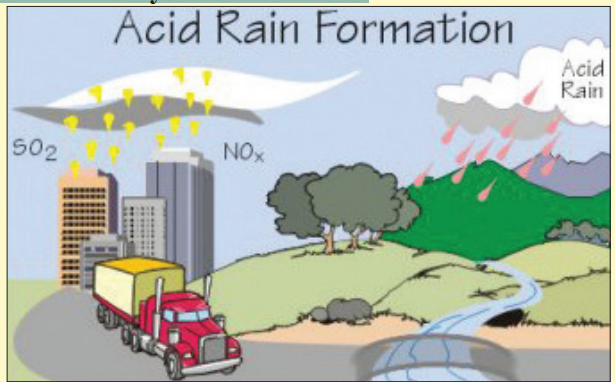
(Photo credit: Environmental Protection Agency (EPA))
Factories produce bad toxic gases called SO2
and NOx and release them
into the air (these are represented by the yellow dots in the diagram).
When SO2and NOx combine with the air, acid rain is created.
Acid rain is caused by a chemical reaction that begins when compounds
like sulfur dioxide and nitrogen oxides are released into the air. These
substances can rise very high into the atmosphere, where they mix and
react with water, oxygen, and other chemicals to form more acidic
pollutants, known as acid rain. Sulfur dioxide and nitrogen oxides
dissolve very easily in water and can be carried very far by the wind. As
a result, the two compounds can travel long distances where they become
part of the rain, sleet, snow and fog that we experience on certain days.
Human activities are the main cause of acid rain. Over the past few
decades, humans have released so many different chemicals into the
air that they have changed the mix of gases in the atmosphere. Power
plants release the majority of sulfur dioxide and much of the nitrogen
oxides when they burn fossil fuels, such as coal, to produce electricity.
In addition, the exhaust from cars, trucks and buses releases nitrogen
oxides and sulfur dioxide into the air. These pollutants cause acid rain.
Acid rain has a pH below 5.6. Normal rain has a pH of about 5.6, which
is slightly acidic. (The pH value is a measure of acidity or alkalinity,
ranging from 0 to 14. A pH measurement of 7 is regarded as neutral.
Measurements below 7 indicate increased acidity, and those above 7
indicate increased alkalinity.)
The principal natural phenomena that contribute acid-producing gases
to the atmosphere are emissions from volcanoes and from biological
processes that occur on the land, in wetlands and in the oceans. The
effects of acidic deposits have been detected in glacial ice thousands
of years old in remote parts of the globe. Principal human sources are
industrial and power-generating plants and transportation vehicles. The
gases may be carried hundreds of miles in the atmosphere before they
are converted to acids and deposited.
Since the industrial revolution, emissions of sulfur and nitrogen oxides
to the atmosphere have increased. Industrial and energy-generating
facilities that burn fossil fuels, primarily coal, are the principal sources
of increased sulfur oxides. These sources, plus the transportation sector,are the major originators of increased nitrogen oxides.
The problem of acid rain has not only increased with population and
industrial growth, it has become more widespread. The use of tall
smokestacks to reduce local pollution has contributed to the spread of
acid rain by releasing gases into regional atmospheric circulation. The
same remote glaciers that provide evidence of natural variability in acidic
deposition show, in their more recently formed layers, the increased
deposition caused by human activity during the past half century.
Effects of acid rain
Acid rain causes acidification of lakes and streams and contributes to
the damage of trees at high elevations (for example, red spruce trees
above 2,000 feet) and many sensitive forest soils. In addition, acid
rain accelerates the decay of building materials and paints, including
irreplaceable buildings, statues and sculptures that are part of our nation’s
cultural heritage. Prior to falling to the earth, sulfur dioxide (SO2
) and
nitrogen oxide (NOx) gases and their particulate matter derivatives—
sulfates and nitrates—contribute to visibility degradation and harm
public health.
Environmental effects of acid rain
The most obvious environmental effect of acid rain has been the loss
of fish in acid-sensitive lakes and streams. Many species of fish are not
able to survive in acidic water. Acid rain affects lakes and streams in two
ways: chronic and episodic. Chronic, or long-term acidification results
form years of acidic rainfall. It reduces the alkalinity (buffering capacity)
and increases the acidity of the water. Chronic acidification may reduce
the levels of nutrients such as calcium, which, over time, may weaken
the fish and other plants and animals in an aquatic ecosystem. Episodic
acidification is a sudden jump in the acidity of the water. This can
result from a heavy rainstorm. It also happens in the spring, because the
sulfates and nitrates will concentrate in the lowest layers of a snowpack.
In the spring, when that snow melts, it will be more acidic than normal.
Episodic acidification can cause sudden shifts in water chemistry. This
may lead to high concentrations of substances such as aluminum, whichmay be toxic to fish

Dead fish in a lake polluted by acid rain
Most of the effects on forests are subtle. Acid deposition may influence
forest vegetation and soils. Acid rain has been cited as a contributing
factor to the decline of the spruce-fir forests throughout the eastern
United States. Acid rain may remove soil nutrients such as calcium
and magnesium from soils in high elevation forests and cause damage
to needles of red spruce. Acid rain may also help weaken the natural
defences of some trees, making them more vulnerable to some diseases
and pests.
Acid rain deposits nitrates that can lead to increases in nitrogen in forests.
Nitrogen is an important plant nutrient, but some forest systems may
not be able to use all they receive, leading to nitrogen saturation. In the
eastern United States, there is evidence of nitrogen saturation in some
forests. Nitrates can remove additional calcium and magnesium from
the soils. Continued nitrogen deposition may alter other aspects of the
nutrient balance in sensitive forest ecosystems and alter the chemistryof nearby lakes and streams

Maize plantation prematurely dried by acid rain
Excess nitrogen may cause eutrophication (over-nourishment) in areas
where rivers enter the ocean. This may lead to unwanted growth of
algae and other nuisance plants. As much as 40% of the total nitrogen
entering coastal bays on the Atlantic and Gulf coasts may come from
atmospheric deposition.
Effects of acid rain - human health
Acid rain looks, feels and tastes just like clean rain. The harm to people
from acid rain is not direct. Walking in acid rain, or even swimming in
an acid lake, is no more dangerous than walking or swimming in clean
water. However, the pollutants that cause acid rain—sulfur dioxide (SO2
)
and nitrogen oxides (NOx)—do damage human health. These gases
interact in the atmosphere to form fine sulfate and nitrate particles that
can be transported long distances by winds and inhaled deep into people’s
lungs. Fine particles can also penetrate indoors. Many scientific studies
have identified a relationship between elevated levels of fine particles
and increased illness and premature death from heart and lung disorders,
such as asthma and bronchitis.
Based on health concerns, SO2
and NOx have historically been regulated
under the Clean Air Act, including the Acid Rain Programme. In theeastern U.S., sulfate aerosols make up about 25 percent of fine particles
By lowering SO2
and NOx emissions from power generation, the Acid Rain
Programme will reduce the levels of fine sulfate and nitrate particles and
so reduce the incidence and the severity of these health problems. When
fully implemented by the year 2010, the public health benefits of the Acid
Rain Programme are estimated to be valued at $50 billion annually, due
to decreased mortality, hospital admissions and emergency room visits.
Decreases in NOx emissions are also expected to have a beneficial impact
on human health by reducing the nitrogen oxides available to react with
volatile organic compounds and form ozone. Ozone impacts on human
health include a number of morbidity and mortality risks associated withlung inflammation, including asthma and emphysema.
Activity 3
Summary writing
In 100 words, describe the causes and effects of acid rain. Compare yoursummaries with those of your classmates.
Describing an industrial process
Using the passive voice and sentence connectors
Activity 1Work
In our previous classes, we learnt about the passive voice. Share with
a classmate what you know about the passive voice and why it is an
appropriate tense for describing processes. You may exhibit knowledge
by writing a simple process of getting a product using the passive tense.Compare your paragraph with those of your classmates.
Activity 2
Sentence connectors
Read these sentences. Share their meaning among yourselves.
1. Science is amusing but also wearying; it is fascinating yet
challenging.
2. Be proud to stand up for purity because that way alone leads to a
magnetic personality.
3. On the other hand, if you do not pay attention to the scientific
details, science can turn out to be harmful.
4. Last month, he was given the final warning against drinking. Even
so, he did not heed the warning and was eventually expelled.
5. We could not allow such a habitual drunkard to join us; besides, he
had already exhibited ill manners that he was bent on not changing.
Note
The words and phrases (sentence connectors) in italics, make connections
between one sentence or clause and another, doing some of the work ofmaking clear the relationship between the ideas expressed.
Activity 3
Work
Study the following sentence connectors. Discuss their meaning and
use them in sentences of your own.
1. Expressing addition: too, also, equally, moreover, additionally
(in addition to), at the same time, in the same way, similarly,
furthermore, as a matter of fact, etc.
2. Expressing cause and effect: as a result, therefore, consequently,
so, thus, because, of that.
3. Expressing comparison: similarly, unlike.
4. Expressing concession: however, nevertheless, and yet, besides, a
the same, through, in spite of (despite), in any case, still, anyway,
even so.
5. Expressing contrast: on the one hand/on the other hand, on thecontrary, however, but, yet, nevertheless, in spite of.
Activity 4
Writing work
Having shared knowledge about the passive tense and sentence
connectors, think of a product whose production process you are familiar
with.
Use the passive tense and sentence connectors to describe the process of
making the product. Compare your descriptive composition with thoseof your classmates
Activity 5
Research
Think of a product. It could be an article of clothing, food product,
beverage or anything else. Visit a factory where that product is made.
Observe the process the product goes through until it is produced. The
guided tour and explanations by the production staff will provide useful
information for your research.
Make notes about every production stage. Write down the process
using the notes you took. Try to present your descriptive essay using
the passive voice.
Write down the steps and share them with your classmates. If there are
any by-products made from the main product, explain them and discusstheir importance
Activity 1
Read the passage below describing the process of
making sugar
Make brief notes and then a summary of the process of making sugar.
Share the notes and summary with other classmates. Before reading, first
study the photographs about the process of making sugar, then matchthem with the story.
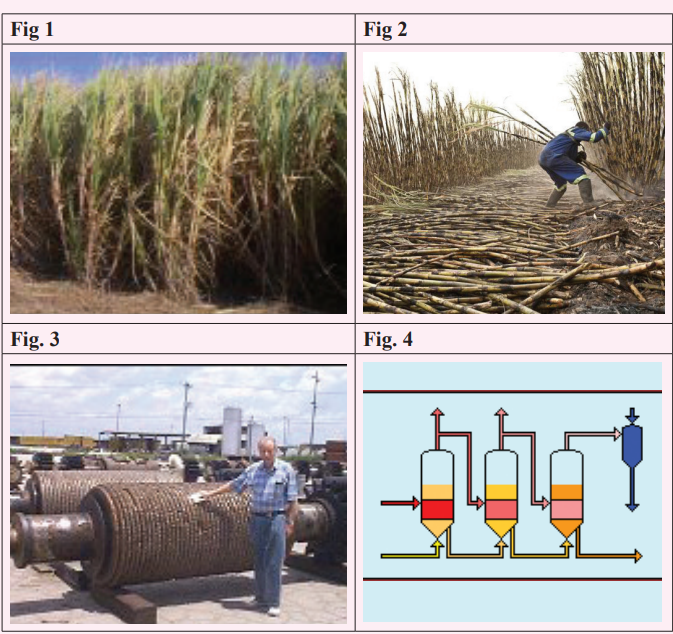
Growing the cane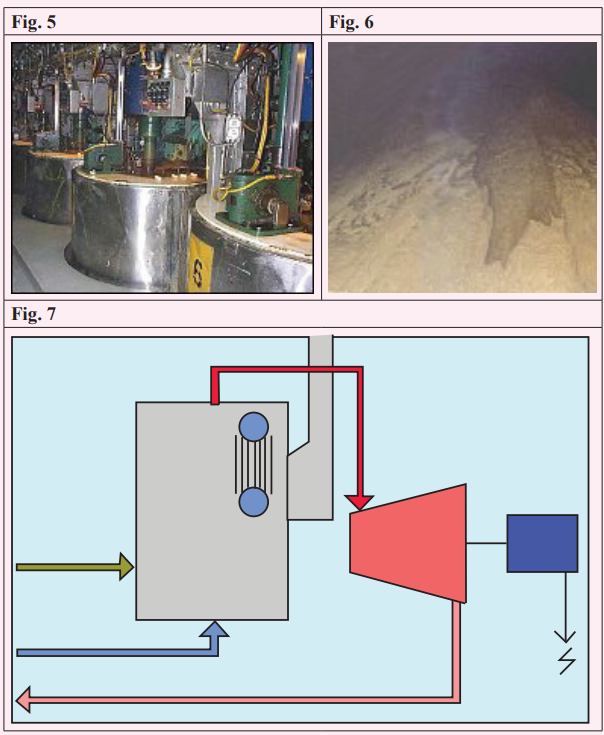
Sugar cane is a sub-tropical and tropical crop that prefers lots of sun and
lots of water – provided that its roots are not waterlogged. It typically
takes about 12 months to reach maturity although the time varies widely
around the world from as short as six months in Rwanda to 24 months in
some places. Where it differs from many crops is that it re-grows fromthe roots so the plant lasts through many cycles.
Harvesting
Sugar cane is harvested by chopping down the stems but leaving the
roots so that it re-grows in time for the next crop. Harvest times tend to
be during the dry season and the length of the harvest ranges from as
little as 2 ½ months up to 11 months. The cane is taken to the factory:
often by truck or rail wagon.
Extraction
The first stage of processing is the extraction of the cane juice. In many
factories the cane is crushed in a series of large roller mills: similar to a
mangle [wringer] which was used to squeeze the water out. The sweet
juice comes gushing out and the cane fibre is carried away for use in
the boilers. In other factories a diffuser is used as is described for beet
sugar manufacture. Either way the juice is pretty dirty: the soil from
the fields, some small fibres and the green extracts from the plant are
all mixed in with the sugar.
Evaporation
The factory can clean up the juice quite easily with slaked lime (a relative
of chalk) which settles out a lot of the dirt so that it can be sent back
to the fields. Once this is done, the juice is thickened up into a syrup
by boiling off the water using steam in a process called evaporation.
Sometimes the syrup is cleaned up again but more often it just goes on
to the crystal-making step without any more cleaning. The evaporation
is undertaken in order to improve the energy efficiency of the factory.
Boiling
The syrup is placed into a very large pan for boiling, the last stage. In the
pan even more water is boiled off until conditions are right for sugar crystals
to grow. You may have done something like this at school but probably
not with sugar because it is difficult to get the crystals to grow well. In the
factory the workers usually have to throw in some sugar dust to initiate
crystal formation. Once the crystals have grown the resulting mixture
of crystals and mother liquor is spun in centrifuges to separate the two,
rather like washing is spin dried. The crystals are then given a final drywith hot air before being stored ready for dispatch.
Storage
The final raw sugar forms a sticky brown mountain in the store and
looks rather like the soft brown sugar found in domestic kitchens. It
could be used like that but usually it gets dirty in storage and has a
distinctive taste which most people don’t want. That is why it is refined
when it gets to the country where it will be used. Additionally, because
one cannot get all the sugar out of the juice, there is a sweet by-product
made: molasses. This is usually turned into a cattle food or is sent to a
distillery where alcohol is made.
Power
So what happened to all that fibre from crushing the sugar cane? It is
called “bagasse” in the industry. The factory needs electricity and steam
to run, both of which are generated using this fibre.
The bagasse is burnt in large furnaces where a lot of heat is given out
which can be used in turn to boil water and make high pressure steam.
The steam is then used to drive a turbine in order to make electricity
and create low pressure steam for the sugar making process. This is the
same process that makes most of our electricity but there are several
important differences.
When a large power station produces electricity it burns a fossil fuel
[once used, a fuel that cannot be replaced] which contaminates the
atmosphere and the station has to dump a lot of low grade heat. All this
contributes to global warming. In the cane sugar factory the bagasse
fuel is renewable and the gases it produces, essentially CO2
, are more
than used up by the new cane growing. Add to that the factory use of
low grade heat [a system called co-generation] and one can see that awell run cane sugar estate is environmentally friendly.
The passive voice
Activity 1
Study the sentence below taken from the readingpassage.
The bagasse is burnt in large furnaces where a lot of heat is given out
which can be used, in turn, to boil water and make high pressure steam.
What do you note about the subject of the sentence above and itsrelationship with the verb?
Activity 2
Writing sentences using the passive voice
Identify 10 sentences in which the passive voice has been used in the
text which you read first. Write them in your exercise book, and discusswith a classmate their meanings.
Describing a mining process
Activity 1
DiscussionDescribe the mining process of minerals
Activity 2
Read the text below carefully.
For each sub-section of a paragraph, write one main sentence to
summarise the paragraph.
Introduction
Tin (Sn) is one of the few metals which have been used and traded
by humans for more than 5,000 years. One of its oldest uses is in
combination with copper to make bronze. Copper was first coated with
tin in the first century AD and tin-plated iron was manufactured in the
16th century. It has the advantageous combinations of a low melting
point, malleability, resistance to corrosion and fatigue, and the ability to
alloy with other metals. It is also non-toxic and easy to recycle.
Occurrence
Cassiterite (SnO2
) is by far the most important tin ore, although small
amounts of tin are recovered from sulphide minerals such as stannite
(Cu2
FeSnS4
). Tin occurs in both primary and secondary deposits. Primary
deposits are typically associated with granite intrusive rocks which form
when magma bodies are embodied into rock beneath the earth’s surface,
rather than on it as in the case of volcanic rock. Primary deposits can
occur within the granite or within pegmatities or aplites (dyke-like
rocks) associated with the granite. They occur also in rocks surrounding
the margins of the intrusive rocks as veins, disseminations, skarns or
carbonate replacements generated by tin-bearing fluids derived from the
granite magmas. Secondary deposits (placers) derive from the weathering
and erosion of primary tin deposits. Cassiterite is chemically resistant,
heavy and readily forms residual concentrations. These concentrations
may develop over a primary deposit (eluvial) and on slopes below the
deposit (colluvial). When the cassiterite reaches a drainage system, it
may be transported to a river channel and concentrated into an alluvialplacer deposit.
A placer deposit buried by younger sediments or lava is known as a deep
lead. Deposits in oceanic submerged river channels are important sources
of tin. More than half of the world’s tin production is from deposits suchas these, mainly in Malaysia, Indonesia and Thailand.
Mining
The main method of mining large placer tin deposits is by bucket-line
dredging. The alluvium containing the tin is excavated and transported
by a continuous chain of buckets to the interior of the dredge where
it is washed and roughly concentrated. In East Africa particularly,
smaller deposits, or those unsuitable for dredging (e.g. because the
bedrock is very rough) are worked by gravel pumping. The alluvium
is broken up by a high pressure jet of water and the resulting slurry is
pumped to the concentrating plant. The impure cassiterite concentrate
is further concentrated by gravity methods which involve passing the
concentrate in a stream of water over equipment such as jigs, spirals,
or shaking tables. This separates the heavy cassiterite from the lighter
minerals such as quartz. Magnetic or electrostatic separation removes
the heavy mineral impurities. The end product is a cassiterite concentrate
containing about 70% tin. Vein and disseminated tin deposits are mined
by the same methods used in hard-rock mining of other non-ferrous ores
such as zinc. The ore is broken by drilling and blasting, transported to a
concentrator where it is crushed and ground and then concentrated by
gravity methods. The concentrate is usually of a lower grade (about 50%
tin) than placer concentrate because of the fine grain size of the cassiterite
and the difficulty of removing all the associated sulphide minerals.
Although flotation is not as efficient for tin ores as it is for sulphide
ores, it is used increasingly to improve the amount of tin recovered and
to recover tin from the residues of earlier treatment.
Smelting
Cassiterite is reduced to tin by heating with carbon at 1200°C to
1300°C. Reverberatory furnaces are used to smelt tin concentrate and,
for additional tin recovery, to re-smelt slag, which is left after the ore
has been smelted.
A furnace charge consists of cassiterite, a carbon-reducing agent, and
limestone and silica fluxes. Smelting takes 10 to 12 hours. The molten
batch is tapped into a settler from which the slag overflows into pots.
The molten tin from the bottom of the settler is cast into slabs or pigs
(of about 34 kg) for refining, and the cooled slag, which contains 10 to25% tin, is crushed and re-smelted.
Refining
Tin produced by smelting concentrate or slag commonly contains
metallic impurities which must be removed by refining before the
tin is marketed. Refining may be by heat treatment or by electrolytic
processes. Heat treatment is the more widely used method and involves
heating the tin from smelters on an inclined hearth to a temperature just
above the melting point of pure tin, but below that of the melting point
of the impurities. The relatively pure molten tin flows into a kettle and
impurities remain behind in a residue which is re-treated to recover more
tin. As there is not a great demand for tin of extremely high purity, the
more costly electrolytic method is rarely used. Tin concentrate sometimes
also contains tantalum and niobium. The concentrate is smelted in an
electric furnace and tantalum and niobium are recovered from the slag.
The tin produced here contains a small amount of antimony and is used
for alloys.
Uses
There are many important uses for tin. Most is used to produce tinplate,
or steel coated with tin which is used for food packaging. Tin and tin
alloys are used also for solder, especially in the electronics industry. It is
commonly used as an alloy for bearing metal and as an alloy in metallic
coatings. Inorganic compounds of tin are used in ceramics and glazes.
Organic compounds of tin are used in plastics, wood preservatives,pesticides and in fire retardants.
Describing the greenhouse effect
Activity 1
Writing and evaluating a text
Carefully read the text below. It is a summary paragraph about
greenhouse gases and their effect.
Greenhouse gases are certain gases in the atmosphere (water vapour,
carbon dioxide, nitrous oxide, and methane, for example) that trap
energy from the sun. Without these gases, heat would escape back into
space and the Earth’s average temperature would be about 60º F colder.
Because of how they warm our world, these gases are referred to asgreenhouse gases.
Activity 2
Research
Now carry out research about the greenhouse effect and write a text
describing its formation process in the atmosphere. Remember to give
a title to your text. Your piece of writing should flow in organised
paragraphs and you should paraphrase your essay. Paraphrasing means
using your own words to write a text, not lifting a sentence, paragraph
or the entire text from the original source of material. Use the text to get
knowledge, but be free to include your own ideas, and factual details to
suit your writing. Present essays of not more than three paragraphs forfurther discussion with your classmates.
