Topic outline
UNIT 1: INTRODUCTION TO ORGANIC CHEMISTRY
Key unit competency
Apply IUPAC rules to name organic compounds and explain types of isomers for
organic compounds
Learning objectives: Students will Should able to:
• Classify organic compounds as aliphatic, alicyclic and aromatic
• Determine different formulae for given organic compounds
• Describe the common functional groups and relate them to the homologous
series
• Use IUPAC rules to name different organic compounds• Describe the isomers of organic compounds
Introductory Activity
Consider the following substances: Sodium chloride, starch, table sugar,
magnesium carbonate, glucose, sodium hydrogen carbonate, water.
1. Heat a small sample of each ( 5g for solids, 5ml for liquids) in a crucible
2. Record your observations.
3. From the observations, classify the substances listed above.
4. What criterion do you use for that classification?5. Interpret your observations
Organic chemistry is defined as the study of the compounds mainly composed by
carbon and hydrogen atoms, and sometimes oxygen, nitrogen, phosphorus, sulphur
and halogens atoms. The study of the rest of the elements and their compounds falls
under the group of inorganic chemistry. However, there are some exceptions such
as carbonates, cyanides, carbides, carbon oxides, carbonic acid, carbon disulphide
which are considered as inorganic compounds. Since various organic compounds
contained carbon associated with hydrogen, they are considered as derived from
hydrocarbons. Thus, a more precise definition of organic chemistry is: “the study ofhydrocarbons and the compounds which could be thought of as their derivatives’’.
The organic and inorganic compounds can be differentiated based on some of theirproperties as summarised in the following table.
Table 1.1 General features of organic and inorganic compounds
Why to study organic chemistry as a separate branch?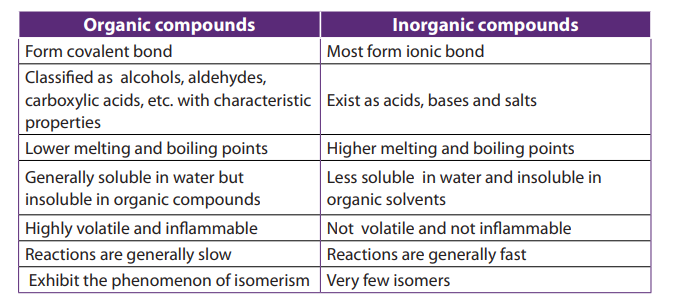
The organic chemistry involves the study of all chemical reactions that are commonly
used in industries and many other organic reactions that take place in living systems.
Materials used in everyday life, food processing and other manufacturing objects are
obtained based on organic chemistry. Some other reasons are highlighted below.
• Large number of compounds: up to now, no one knows exactly the number
of organic compounds that are present in nature.
• Built of relatively few elements: The elements frequently encountered
in organic compounds are carbon, hydrogen, oxygen, nitrogen, sulphur,
phosphorous, and halogens;
• Unique characteristic of carbon to undergo catenation: carbon atom is
unique among other elements whose atoms possess the capacity to unite
with each other by the covalent bonds resulting in a long chain of carbons ( i.e:
polysaccharides, proteins, polyesters, polyamides…).
Isomerism is the existence of compounds that have the same molecular formula
but different arrangements of atoms; these compounds are called “isomers”.
• Functional groups as basis of classification: Organic molecules contain
active atoms or groups of atoms which determine their chemical behaviour.
These are called functional groups joined in a specific manner. Therefore,
organic compounds with similar functional groups display similar propertiesand form a class.
• Combustibility: organic compounds are combustible.
• Nature of chemical reactions: organic compounds being formed by covalentbonds, they are slow and often have a low yield.
Importance of organic chemistry
The organic chemistry is a subject that plays an important role in modern life. In
general, there is no art, science or industry where knowledge of organic chemistryis not applied.
Examples where organic chemistry is applied:
1) Application in daily life.
In our day-to-day life, we find many substances or materials that are commonly used
and the later are made of organic compounds.
• Food: starch, fats, proteins, vegetables,...
• Clothes: cotton, wool, nylon, dacron, ....
• Fuels: petrol, diesel oil, and kerosene
• Dyes of all kinds
• Cosmetics (body lotion,…)
• Soaps and detergents
• Medicine: cortisone, sulphonamide, penicillin,…
• Drugs: morphine, cocaine,...
• Stationery: pencils, paper, writing ink,…• Insecticides, rodenticides, ovicides …
2) Applications in industry
The knowledge of organic chemistry is required in many industries such as
manufacture of food, pharmacy, manufacture of dyes and explosives, alcoholindustry, soil fertilisers, petroleum industry, etc.
3) Study of life processes
Organic chemistry in other words is the chemistry of life. For example the vitamins,
enzymes, proteins and hormones are important organic compounds produced inour body to ensure its proper development.
1.1. Classification of organic compounds

Organic compounds are classified as: aliphatic, alicyclic and aromatic (Figure 1.1)
(https://chemistry.tutorvista.com/organic-chemistry/hydrocarbons.html) ;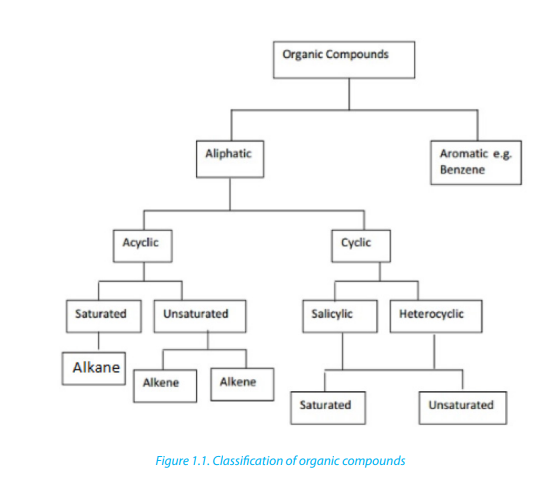
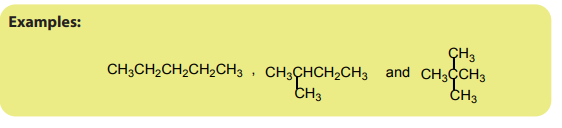
1.1.1. Aliphatic compounds
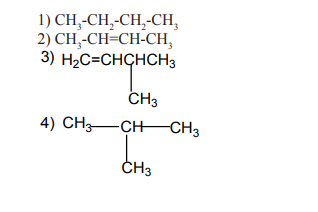
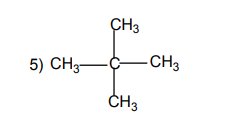
Aliphatic compounds are organic compounds in which the carbon atoms are
arranged in a straight or branched chain.Examples
1.1.2. Alicyclic compounds
Alicyclic compounds are organic compounds that contain one or more carbon rings
that may be saturated or unsaturated.Example: 1) cyclobutane
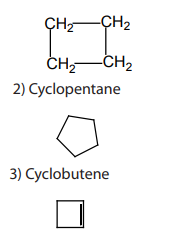
1.1.3. Aromatic compounds
Aromatic compounds are compounds that contain a closed ring that consists of
alternating single and double bonds with delocalised pi electrons.Example:
Aromatic compounds are designated as monocyclic, bicyclic and tricyclic if they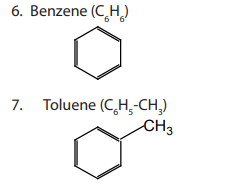
contain one, two or three rings, respectively.
Examples:
Note: Heterocyclic compounds: Are also classified as cyclic compounds which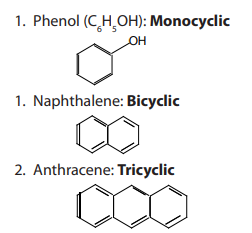
include one or two atoms other than carbon (O, N, S) in the ring.Thus furan, thiopheneand pyridine are heterocyclic compounds.
Checking up 1.1: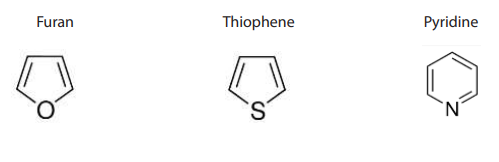
Observe the following compounds and classify them as aliphatic, alicyclic andaromatic.

1.2. Types of formulas for organic compounds
Activity 1.2
1. Explain the terms empirical, molecular and structural formulae.
2. Use examples of organic compounds to differentiate the types of theformulae above.
Atoms bond together to form molecules and each molecule has a chemical formula.
In organic chemistry, we can distinguish empirical, molecular and structural formulas.
1.2.1. Empirical formula
The empirical formula is the simplest formula which expresses the ratio of the
number of atoms of each element present in a particular compound. The empirical
formula is determined using the percentage composition according to the
following steps.
i. The percentage of each element, considered as grams of that element in
100g of the compound, is divided by its atomic mass. This gives the number
of moles of the element in 100g of the compound.
ii. The result in i. is then divided by the lowest ratio (number of moles in 100g
of the compound), seeking the smallest whole number ratio.
iii. If the atomic ratios obtained in ii. are not the whole number, they should
be multiplied by a suitable common factor to convert each of them to the
whole numbers (or approximatively equal to the whole numbers). Minorfractions are ignored by rounding up or down (ex: 7.95 = 8).
Example
An analysis of organic compound showed that it has 39.13% carbon, 52.23%
oxygen and the remaining is hydrogen. Determine the empirical formula of thecompound.
Note: 2.65 can not be adjusted to 3 and it is multiplied by 3 equals to 7.95 which
is rounded to 8.
1.1.2. Molecular formula
The molecular formula is a formula expressing the exact number of atoms of each
element present in a molecule.Molecular formula = Empirical formula x n
Note: When n = 1, the molecular formula is the same as the empirical formula.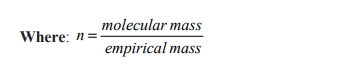
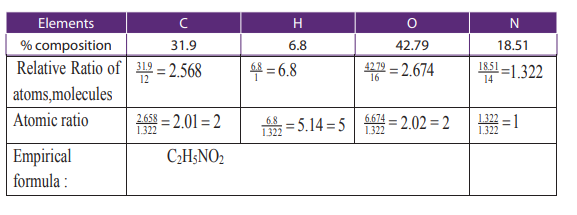
Example 1:
An organic compound contains 31.9% by mass of carbon, 6.8% hydrogen and 18.51%
nitrogen and the remaining percentage accounts for oxygen. The compound has avapour density of 37.5. Calculate the molecular formula of that compound.
Answer:
Vapour density = a half molecular mass
Molecular mass = 2 x vapour density = 2 x 37.5 = 75g/mol
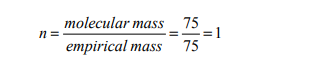
Hence the molecular formula = empirical formula

Example 2:
0.45g of an organic acid on combustion gave 0.44g of carbon dioxide and 0.09gof water. If the molecular mass of the acid is 90, deduce the molecular formula.
Answer:
• Percentage of carbon in CO2 : x x100 =26.66%
• Percentage of hydrogen in H2 O: x x100 =2.22%• Percentage of oxygen = 100 – (26.66 + 2.22) = 71.12%
Note: From the above calculations, we can extend our generalized expression:
% of Oxygen = 100 – (% hydrohen + % carbon)
Finding formulae by combustion method
the formula of a hydrocarbon can be found from the results of combustion experiment.
A hydrocarbon in vapour phase is burned more than oxygen to form carbon dioxide
and water vapour. When the mixture of gases is cooled to room temperature, water
vapour condenses to occupy a very small volume. The gaseous mixture consists of
carbon dioxide and unused oxygen. The volumes of carbon dioxide can be found by
absorbing it in an alkali. From the volumes of gases, the equation for the reactionand the formula of hydrocarbon can be found.
The combustion method can be used for other compounds also, e.g. ammonia
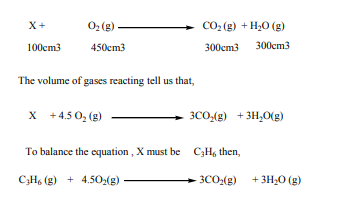
Example 1:
when 100cm3 of hydrocarbon X burn in 500cm3 of oxygen, 50cm3 of oxygen are
unused, 300cm3 of carbon dioxide are formed and 300cm3 of steam are formed.Deduce the equation for the reaction and the formula of the hydrocarbon
Answer:
Example 2:
10cm3 of a hydrocarbon, Ca Hb, are exploded with an excess of oxygen.
A contractionb of 35cm3
occurs, all volumes being measured at room temperature and pressure.
On treatment of the products with sodium hydroxide solution,
a contraction of 40cm3 occurs. Deduce the formula of the hydrocarbon

1.2.3. Structural formulas
Structural formula shows how the different atoms in a molecule are bonded
(i.e. linked or connected)
There are three types of structural formulas: displayed, condensed and skeletal(stick) formulas.
Example:
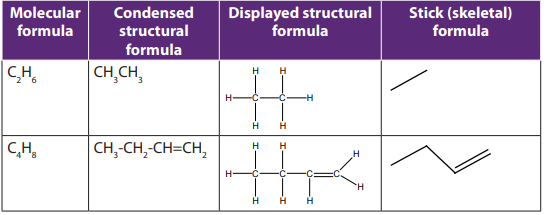
Note: Stick formula is also considered as structural formula.
1.3. Functional groups and homologous series1.3.1 Functional groups
Activity: 1.3.1
Using books or internet, explain the term functional group and point out somecommon functional groups.
A functional group is an atom or group of atoms in a molecule which determines
the characteristic properties of that molecule. Examples of some fuctionnal groupsare indicated in the Table 1.2.
Table 1.2: Name and give examples of functional groups in organic compounds

1.3.2. Homologous series
Activity 1.3.2.
By doing your own research, provide the meaning of “homologous series”. What
are the characteristics of such a series? Illustrate your answer by using examplesof alkanes, alcohols, carboxylic acids.
When members of a class of compounds having similar structures are arranged in
order of increasing molecular mass, they are said to constitute a homologous series.
Each member of such a series is referred to as a “homologous” of its immediate
neighbours. For example, the following sequence of straight chain of alcohols formsa homologous series.
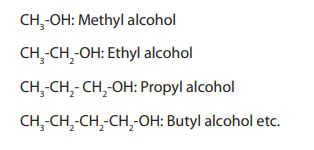
Characteristics of a homologous series
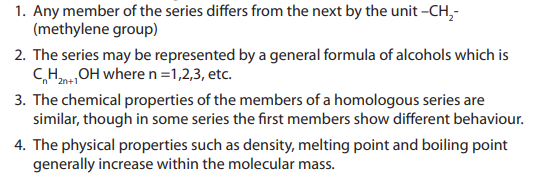
Checking Up 1.3
1.4. General rules of nomenclature of organic compounds
according to IUPAC
Activity 1.4.
By your own research, describe the rules that are applied to name the organic compounds.Your answers can be given as a form of a report.
The organic compounds are named by applying the rules set by the International
Union of Pure and Applied Chemistry (IUPAC). The purpose of the IUPAC system of
nomenclature is to establish an international standard of naming compounds tofacilitate the common understanding.
In general, an IUPAC name has three essential parts:
• A prefix that indicates the type and the position of the substituents on the
main chain.
• The base or root that indicates a major chain or ring of carbon atoms found
in the molecule’s structure. e.g. Meth- for one carbon atom, eth- for 2 carbon
atoms, prop- for 3 carbon atoms, hex- for five carbon atoms, etc.
• The suffix designates the functional group.
Example -ane for alkanes, -ene for alkenes, -ol for alcohols, -oic acid for carboxylic
acids and so on.
Steps followed for naming organic compounds:
1. Identify the parent hydrocarbon:It should have the maximum length, or the longest chain.
Example

3. Identification of the side chains.
Side chains are usually alkyl groups. An alkyl group is a group obtained by a
removal of one hydrogen atom from an alkane. The name of alkyl group is obtainedby replacing -ane of the corresponding alkane by –yl (Table 1.3).
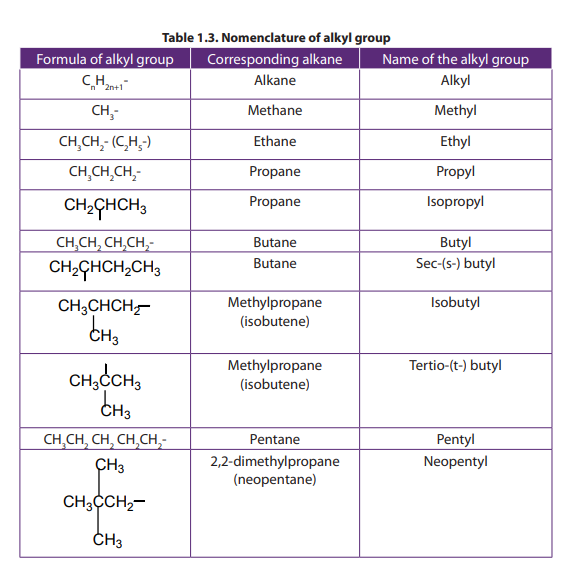
A side chain must be identified by the smallest possible numbers
4. If the same substituent occurs two or more times, the prefix di, tri,tetra, ...is
attached to substituent’s name. Its locants separate the prefix from the name ofthe substituent.
5. Identify the remaining functional groups, if any, and name them. Different side
chains and functional groups will be listed in alphabetical order.
The prefixes di, tri, tetra,...are not taken into consideration when grouping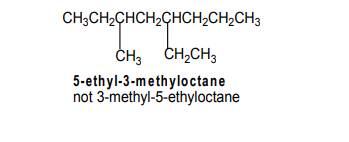
alphabetically. But prefixes such iso-, neo- are taken into account.
Example:
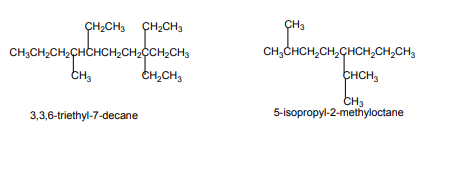
Identify the position of the double/triple bond.
Example:
Number the chain (left to right) or right to left).
The sum of the numbers which show the location of the substituents is the possible smallest.

The correct name will be the one which shows the substituents attached to the third
and fifth carbon, respectively and not to the fourth and the fifth carbon atom.
Numbers are separated by commas Hyphens are added between numbers andwords. Successive words are merged in one word.
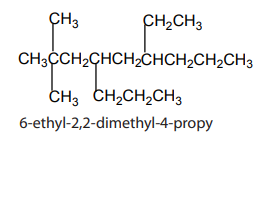
Checking up 1.4
1. Complete the sentence; the systematic nomenclature of organic
compounds follows rules established by the ……………………………
…..............................
2. What are the main parts which made up the name of an organic
compound?3. Name each of the following compounds using the IUPAC system.
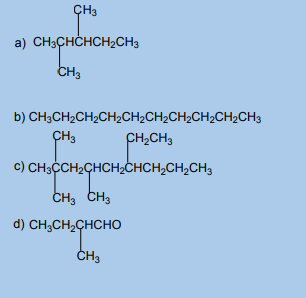
1.5. Isomerism in organic compounds
Activity 1.5:
Consider the following set of compounds:
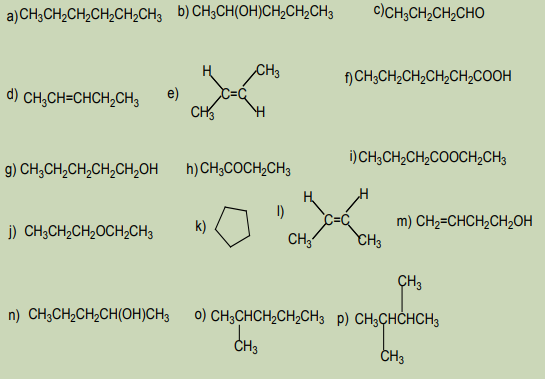
Analyze the structure of the compounds listed above and point out:
1. Compounds with the same structural formula
2. Compounds with the same molecular formula
3. Are there any compounds having the same molecular formula but
different by their structures? Explain the main differences displayed by
them? Name the relationship between them?4. Explain the behavior of the identified in 3)
Isomerism is the existence of compounds that h ave the same molecular formula
but different arrangements of atoms; these compounds are called “isomers”.
Isomers have different physical or/and chemical properties and the difference may
be great or small depending on the type of isomerism.There are two main classes of isomerism: Structural isomerism and stereoisomerism.
1.5.1. Structural isomerism
Activity 1.5.1
1. Referring to the previous activity 1.5 above, what is the relationship
between compounds: a), o and p) in the list of the activity 1.5?
2. Identify the relationship between compounds b) and g) in the activity 1.5?
3. Relate the relationship between compounds: b) and J) in the activity 1.5?
4. Identify the relationship between compounds c) and h) in the activity 1.5?
5. Investigate if there is a relationship between compounds d) and k) in theactivity 1.5?
Structural isomers are compounds with the same molecular formula but withdifferent structural formula.
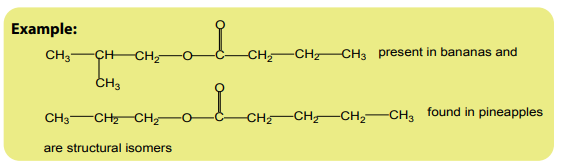
1. Position isomerism
Position isomers are compounds with the same molecular formula but differentpositions of the functional group or substituent(s).
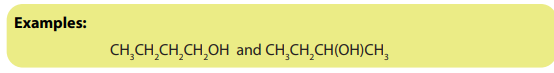
2. Chain isomerism
Chain isomers are compounds with the same molecular formula, belonging to thesame homologous series, with chain of carbon atoms of different length.
3. Functional isomerism
Functional (group) isomers are compounds which have the same molecular formulabut different functional groups.

1.5.2. Stereoisomerism
Activity 1.5.2
1. What is the relationship between compounds e) and l) in the activity 1.5. ?
2. Suggest examples of other organic compounds which have a similarrelationship.
1. Geometrical isomerism
Geometrical isomers or cis-trans isomers are compounds with the same molecular
formula, same arrangement of atoms but differ by spatial arrangements.
This type of isomers is mainly found in alkenes due to the restricted rotation around
the carbon-carbon double bond.
Note: For more information, visit the website below. (https://www.youtube.com/
watch? v=7tH8Xe5u8A0).
The necessary condition for an alkene to exhibit geometrical isomerism is that eachcarbon doubly bonded has two different groups attached to it.

2. Optical isomerism
Activity 1.5.3.
1. Look at your two hands or the Figure 1.2 and discuss the relationship
between them?
2. What are the necessary conditions for such pairs of organic compounds
to exhibit that relationship?3. What name is given to such compounds?
Optical isomers are compounds with the same molecular formula and arrangements
of atoms but have different effect on the plane polarised light.
• A compound that rotates the plane polarised light is said to have an optical
activity.
• This type of isomerism occurs in compounds containing an asymmetric
(asymmetrical) carbon atom or chiral centre1
• When a molecule has chiral centre, there are two non superimposable isomers
that are mirror images of each other.• Such compounds are called enantiomers.
In a mirror, the left hand is the image of the right hand and they are non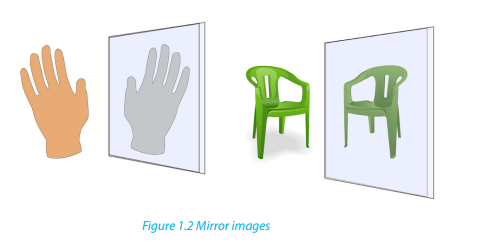
superimposable, i.e. they are enantiomers. An achiral object is the same as its mirrorimage, they are nonsuperimposable.

Checking Up 1.5
1. What is meant by “isomers”
2. Using examples, distinguish structural isomers and stereoisomers.
Describe the sub-classes of each type of isomers.
3. Explain how the nature of the C=C bond gives rise to cis-trans isomerism.
4. Identify which of the isomers of hexane exhibit geometrical isomerism.
5. Which of the following compounds can exist as optical isomers? Justifyyour answer.

6. Give examples of items which are enantiomers.
1.6. End unit assessment
1. a). An atom or group of atoms which determines the characteristic properties
of an organic compound is…………………………………………………..
b). A set of compounds that have the same functional group is referred
as ………………
c). An organic compound that rotates the plane polarized light is said to
be……………….
2. Chain isomers belong to the same class. True/False
3. Organic compounds belonging to the same class have similar physical
properties. True/False4. What is the name of the following compound?

A). 1,1-butyl-2- mthylpropane
B). 2,2,4-trimetylpentane
C). 2,2,4-methylpentane
D). 2,4,4-trimethylpentane
E). none of the previous answer
5. The compound that follows belongs to which class of organic compounds?

A). alcohols
B). alkenes
C). alkynes
D). aromatic
6. The compound that follows belongs to which class ofcompounds?
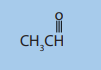
A). ethers
B). aldehydes
C). ketones
D). alcohols
7. Write the structural formula of:
a. 4-ethyl-3-methylheptane
b. 3-ethyl hexane
c. 3,3,5-trimethyloctane
d. 4-ethyl-2,2-dimethylnonane
8. Consider the following compound.
a. Determine the percentage composition of each element present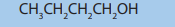
in the compound.
b. Determine the empirical formula of the above molecule
c. From the results from a) calculate the molecular formula of the
compound
d. Write all possible structural formulae of isomers of the compound.
e. Name the isomers in d) according to the IUPAC system.
f. (i) From the results in d) classify the isomers as chain, position,
functional and optical isomers.
(ii) From the results in d) show the compound that can exhibitoptical isomerism.
UNIT 2: ALKANE
Key unit competency
Relate the physical and chemical properties of the alkanes to the preparation
methods, uses and isomerism.
Learning objectives
• Name straight chain alkanes up to carbon-20
• Define homologous series
• Use IUPAC system to name straight and branched alkanes
• Describe the preparation methods of the alkanes
• Prepare and collect methane gas
• Respect of procedure in experiment to carry out preparation of methane or
propane
• Describe and explain the trend in physical properties of homologous series of
alkanes
• Be aware of the dangers associated with combustion reactions of the alkanes
• Write reaction for free radical mechanism for a photochemical reaction
• State the chemical properties of the alkanes
• Develop practical skills,interpret results make appropriate deductions.
• Appreciate the importance of the alkanes in daily life
• Appreciate the dangers caused by the alkanes to the environment as major
sources of air contaminants
• State the uses of the alkanes
Introductory activity
Analyze the picture below and answer to the proposed questions

a. Explain the process observed in the above picture
b. What is the source of the gas produced as shown by the picture?
c. Analyse the environmental problems caused by gas observed in the
picture and suggest different ways to solve it.
Alkanes are the simplest class of organic compounds. They are made of carbon and
hydrogen atoms only and contain two types of bonds, carbon-hydrogen (C-H) and
carbon-carbon (C-C) single covalent bonds. They do not have functional groups.
Alkanes form a homologous series with the general formula where n is the
where n is the
number of carbon atoms in the molecule. The first member of the family has the
molecular formula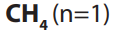 and is commonly known as methane and the second
and is commonly known as methane and the second
member with molecular formula is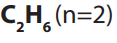 is called ethane.
is called ethane.
These compounds are also known as saturated hydrocarbons. This name is more
descriptive than the term “alkane’’ because both their composition (carbon and
hydrogen) and the fact that the four single covalent bonds of each carbon in their
molecules are fully satisfied or ‘’saturated’’.
The name alkane is the generic name for this class of compounds in the IUPAC
system of nomenclature. These hydrocarbons are relatively unreactive under
ordinary laboratory conditions, but they can be forced to undergo reactions by
drastic treatment. It is for this reason that they were named paraffins (Latin parumaffinis = little activity).
2.1. Nomenclature of alkanes
Activity 2.1
4. Discuss IUPAC rules for naming straight and branched alkanes.
5. Draw the structure of the following compounds:
a. 3-ethyl-4-propyloctane
b. 4-ethyl-2-methylhexane
c. 2,2-dimethylpentane
IUPAC Rules for the nomenclature of alkanes
a. Find and name the longest continuous carbon chain.
b. Identify and name groups attached to this chain.
c. Number the chain consecutively, starting at the end nearest a substituent
group.
d. Designate the location of each substituent group by an appropriate
number and name.
e. Assemble the name, listing groups in alphabetical order. The saturated
hydrocarbon form homologous series (series in which members have similar
chemical properties and each differs from the preceding by a methylene
group
The first four members are known by their common names, from C5
and above the Roman prefixes indicating the number of carbon atoms is written
followed by the ending “ane” of the alkanes.
Note: Alkyl groups are obtained when one hydrogen atom is removed from alkanes;
therefore their names are deduced from the corresponding alkanes by replacing“ane” ending with “yl” desinence (Table 2.1).
Table 2.1. Naming straight chain alkanes

Note: n is the number of carbon atoms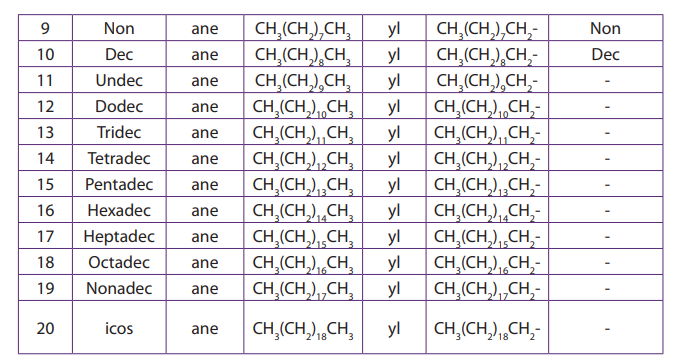
Prefixes di, tri, tetra, sec, tert, are not considered when alphabetizing.
f. In case of chains of the same length, the priority is given for part wheremany branched of alkyl groups appear.
g. For cyclanes or cycloalkanes, the prefix “cyclo” is recommended, followed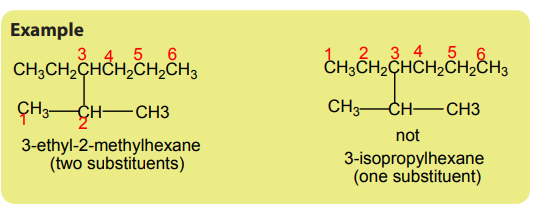
by the name of the alkanes of the same carbon number.But in case of ramified cyclanes, the priority is for the ring.
The organic compounds comprise aliphatic compounds that can be acyclic or
cyclic named respectively as alkanes and cyclanes.
Note: If there are more than one substituent, the numbering is done so that the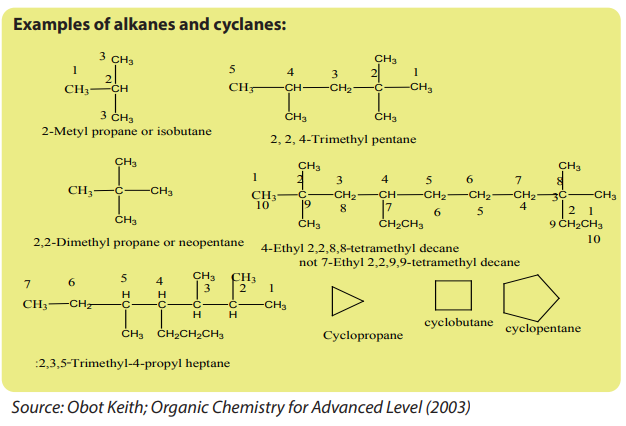
sum of the numbers used to locate the locants is minimum. This is the lowest sumrule.
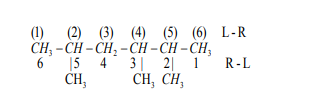
The longest chain has 6 carbons, it is a hexane chain. The sum of locants
Since the sum of the locants for R-L numbering is minimum, then it is preferred.
h. The name of alkane is given by the numbers of the locants (2,3,5-) followed
by the prefixed substituent (trimethyl), followed by the name of the long chain(hexane): 2,3,5-trimethylhexane.
Checking up 2.1
1. What are alkanes? Why are they called saturated hydrocarbons?2. Name each of the following alkanes according to the IUPAC system.
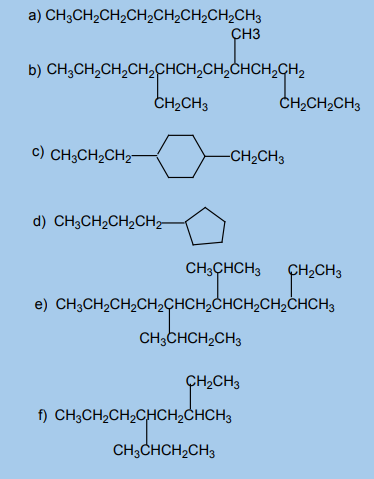
2.2. Isomerism
Activity 2.2.
Identify and write down all the structural formulas that fit the molecularformula
Alkanes show structural isomerism. The easiest way to find isomers is to draw the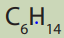 and classify them into the position and chain isomers.
and classify them into the position and chain isomers.
longest chain of carbon atoms first and then reduce it by one carbon first untilrepetition begins to occur.
-Putting the methyl group on position 1 or 5 gives you the same straight chain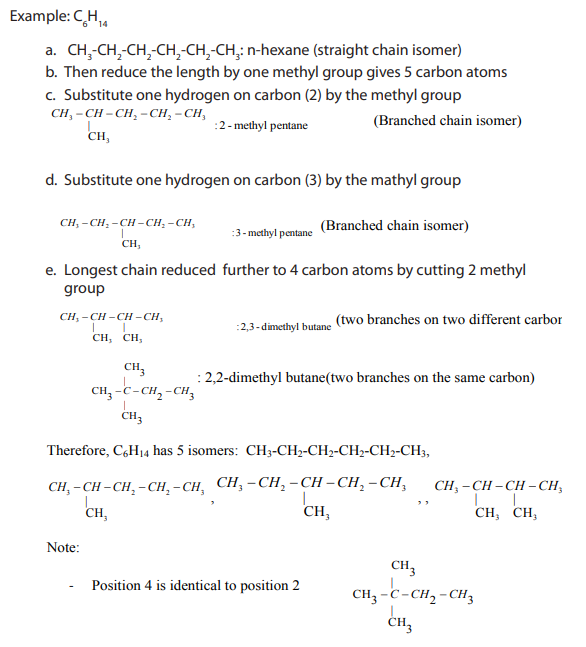
isomer.
Checking up 2.2
Write the structural formulae of all isomers which fit the molecular formula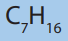
and name each of them according to the IUPAC system.
2.3 Occurrence of Alkanes
Activity 2.3:
Some organic compounds are found in living beings whereas others are
synthesised by humans. Under which category do alkanes fall? Justify youropinion.
1. The alkanes exist in nature in form of natural gases and petroleum. Natural
gas and petroleum existence are the results of decomposition of died bodies
after many years ago.
2. The most natural gas is found in lake Kivu as methane gas but in form of
traces like ethane, propane and butane.
3. Petroleum is one of the largest source of energy in the work. It is formed by
decomposition by bacteria for millions of years died marine living things
and as the last product is petroleum and natural gases which are separated
in fractional distillation of their crude oil and the results are obtainedaccording to their boiling point.
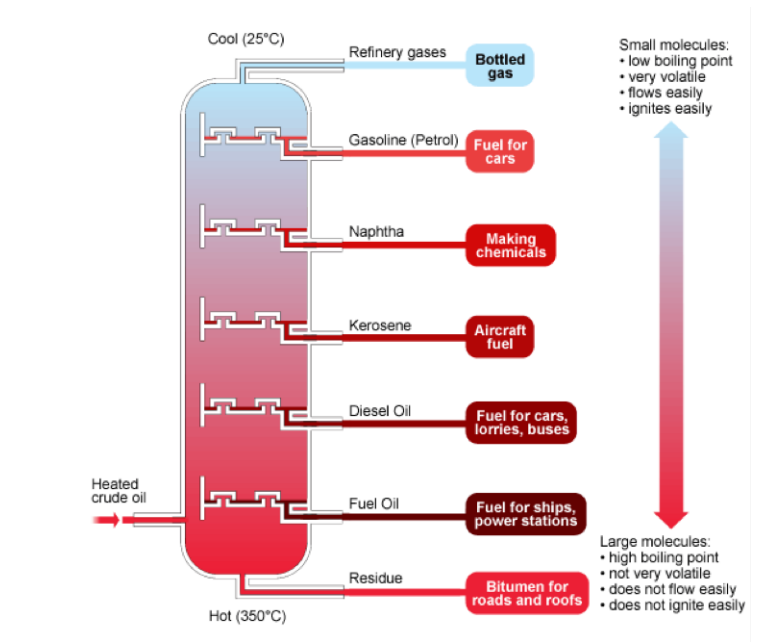
Figure 2.1. Fractional distillation of petroleum
Source:www.bbc.uk/schools/gcse/fractionaldistillationofcrudeoil ;
The fractional distillation and the different fractions are summarized in the followingtable and in the Table 2.2.
Table 2.2. Fractions of crude petroleum

Checking up 2.3:
What are the main sources of alkanes?
2.4. Preparation of alkanes
Activity 2.4
Laboratory preparation of methane gas
Requirements:
Stand and accessories
Delivery tube
NaOH(s)
Sodium acetate(s)
Calcium oxide(s)
Procedure:Set up the apparatus as shown on the diagram below
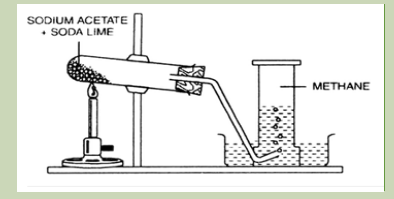
Figure 2.2 Laboratory preparation of methane
Source: https.www. zigya.com/study/book
Prepare a mixture of the reagents in ratio 1:1. Weigh about 3 grams of sodium
acetate and the same quantity as soda lime. Mix them thoroughly in a beaker.
Place about 4 grams of the mixture into a boiling tube.
Seal the boiling tube with a stopper with a gas-delivery tube. The gas-delivery
tube should look upwards.
Fix the boiling tube on a stand.
Heat the test-tube gently with the cold part of the flame. To avoid local
overheating keep the flame in motion.
After a while the gas starts liberating.
Prepare an empty test-tube. Collect some gas keeping this test-tube on top of
the gas delivery tube.
Methane is a flammable gas. To set it on fire turn the covering test tube and
hold a burning match to the end of the gas delivery tube.
The gas burns with a blue (red) fire.
Methane can be prepared by the reaction between sodium acetate and sodium
hydroxide solid according to the equation:
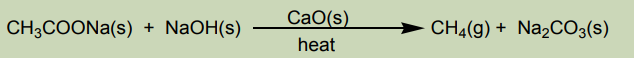
It is collected by the downward displacement of water.
Other gaseous alkanes can be prepared in the same way according to the generalequation.
Note: The reaction is practically used to reduce by one carbon the length of carbon
chain. It is referred as decarboxylation of sodium carboxylates.
Other reactions used for the preparation of alkanes are the following:
1. Addition reaction of hydrogen to alkenes and alkynes in the presence of
catalyst like Nickel, Palladium or platinum produces alkanes: this reaction
is called hydrogenation reaction of alkenes and alkynes; it is also called areduction reaction of alkenes and alkynes.
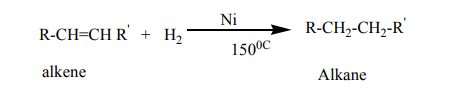
N.B: In organic chemistry, reduction reaction is the reaction that results in
increasing of hydrogen content in the new product.
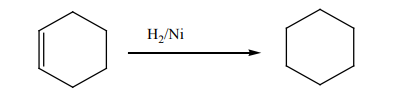
[Hydrogen content in the product
 is higher than the hydrogen content in the reactant
is higher than the hydrogen content in the reactant
Note: Reduction with Platinum and Palladium as catalyst occurs at room
temperature, while using Nickel requires a temperature of about

2. From halogenoalkanes or Alkyl halides
On reduction of alkyl halides with Zn and concentrated hydrochloric acid, alkyl
halides are converted to alkanes.
Checking up 2.4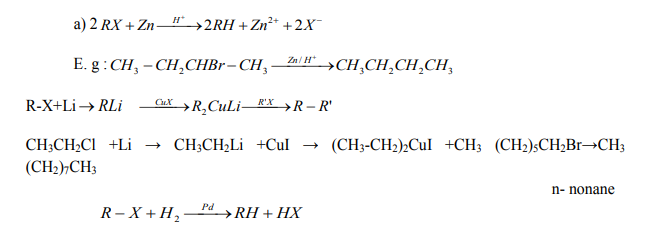
Describe the main reactions used in the preparation of alkanes.
b) Alkyl halides when heated with sodium metal in ether solution give higher
alkanes (alkanes with more carbon atoms) (Wurtz reaction).
Note: This reaction is practically useful in organic synthesis to increase the length of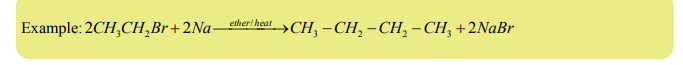
carbon chain.
c) When Alkyl halides are treated with Zn-Cu couple, in the presence of ethanol,
alkanes are formed.
Note: Zn-Cu couple is obtained by adding Zinc granules in aqueous copper (II)sulphate solution where copper is deposited on the Zn pieces.

3. From carbonyl compounds
Reduction of carbonyl compounds, with amalgamated Zinc (alloy made of zincand mercury) and HCl. This is the Clemmensen reduction).
Note: Under special conditions, reduction also is realized by use of H2 and Raney
Nickel or using hydrazine (NH2NH2) and KOH.This is calledWolf Kushner reduction
2.5. Physical properties of alkanes
Activity: 2.5
1. Put 5 ml of hexane in a test tube. Add 5ml of water and shake.
2. Record your observations
3. Repeat the above procedure using,
4. (i) cyclohexane
5. (ii) heptane
6. Repeat the steps 1-3 using carbon tetrachloride instead of water.
7. Record your observations.
8. Search about the melting and boiling point values of the alkanes used inthe experiments above and record your findings.
Melting and boiling points
The values of melting, boiling points, density and physical state of some alkanesare summarized in the table below.
Table 2.3 Physical properties of alkanes
The above Table shows that the boiling and melting points of homologue alkanes
increase with the number of carbon i.e. molecular mass.
Explanation:
The boiling and melting points depend on the magnitude of the Van Der Waal’s
forces that exist between the molecules. These forces increase in magnitude with
molecular mass.
Note: Branched chain isomers have lower boiling and melting points than their
straight chain isomers, because straight chain isomers are closely packed than thebranched chain isomers.
Boiling points decrease with increase in branching because increased branching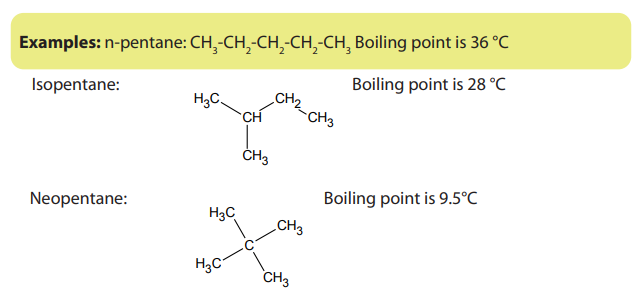
gives the molecule a more nearly spherical shape and this reduces the extent of
contact between neighboring molecules, in other words the branched isomers are
less packed than straight chain isomers, and hence the attractive force betweenthe molecules are reduced hence decrease in the boiling points.
Alkanes are not soluble in water, because of their low polarity and also because
of their inability to form hydrogen bonds. They are, however soluble in non polar
solvents, like benzene, and are miscible with one another.
benzene, and are miscible with one another.
Checking up 2.5
Using data in the Table 2.3, plot a graph of boiling and melting points against
the number of carbon atoms, explain the shapes of the graphs drawn.2.6. Chemical properties of alkanes
Activity 2.6.1
Experiment to investigate the reactivity of alkanes
1. Put 5ml of hexane in a test tube.
2. Add drop wise 5ml of potassium hydroxide and shake
3. Repeat steps 1-2 using bromine water instead of potassium hydroxide
4. Repeat steps 1-3 using octane.5. Record all your observations in the table below.
For a positive test put “yes” and “no” for a negative result.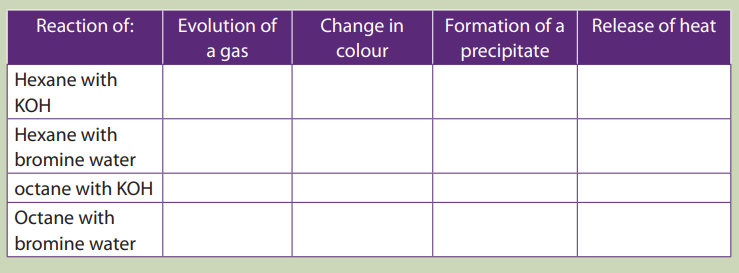
6.What do you deduce from your observations?
Generally, alkanes are quite inert towards common reagents because:
• The C-C bond and C-H bonds are strong and do not break easily.
• Carbon and hydrogen have nearly the same electronegativity valuehence
• C-H bond only slightly polarized; generally C-H bond is considered as covalent.
• They have unshared electrons to offer.They, however, undergo the following reactions.
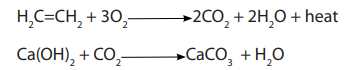
1. Reaction with oxygen
Alkanes react with oxygen to produce carbondioxide and water. However, if oxygenis insufficient carbonmonoxide gas and water are formed.
Carbon dioxide
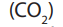 produced from the burning of alkanes or fossil fuels for
produced from the burning of alkanes or fossil fuels for
heating, transport and electricity generation is the major atmospheric pollutant that
increases the green house potential of the atmosphere. Carbon dioxide is the major
Green House Effect (GHE) gas.
Burning wood and forests produce also carbon dioxide and lead to the increase of
that gas in the atmosphere. Methane as another GHE gas is produced by humanactivities, agriculture (Rice), and cattle-rearing.
Activity 2.6.2
Carry out research and discuss different ways of avoiding or reducing theproduction of GHE gases such as carbon dioxide.
There are many natural ways of reducing atmosphere carbon dioxide:
i. Water in seas dissolves millions of tonnes of gas (but less now than it did in
the past, since the average ocean temperature has increased by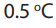
in the last 100 years, and gases are less soluble in hot than in cold water).
ii. Plankton can fix the dissolved carbon dioxide into their body mass by
photosynthesis
iii. Trees fix more atmospheric carbon dioxide than do grass and othervegetation through photosynthesis according to the equation below.
iv.
There are other ways than natural ways of reducing GHE gases and among them
there are the use of technologies that reduce the green house gas emissions, therecycling of the GHE.
Notice: (i) reacts as
reacts as  but slowly while iodine hardly reacts.
but slowly while iodine hardly reacts.
Notice: (i) reacts as
reacts as  but slowly while iodine hardly reacts , Fluorine, the most
but slowly while iodine hardly reacts , Fluorine, the most
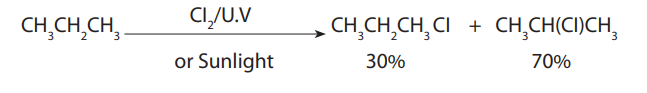
electronegative element of the periodic table reacts with alkanes to givecoke, i.e. a decomposition reaction:
(ii) Due to radical formation involved, the main product of reaction is the one
from the most stable radical, starting with tertiary, secondary, primaryand methyl in decreasing order of stability.
A tertiary free radical is better stabilised by the electron donating methyl groups
than the secondary, primary and methyl ones where the carbon atom is attached tomore hydrogen atoms
3. Dehydrogenation of alkanes gives alkenes under heat and a catalyst like
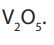
4. Cracking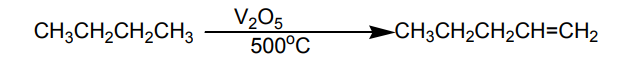
On heating or in the presence of a catalyst, large molecules of alkanes are decomposed
into smaller alkanes and alkenes. If the cracking is performed on heating, it is referredas themocracking.
If the cracking is performed using a catalyst; it is referred as catalytic cracking andmany products result from one reactant as shown below.
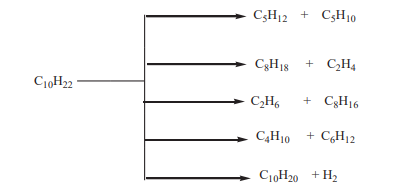
Checking up 2.6
1. Using a series of chemical equations, show how butane can be prepared
from bromoethane
2. Kerosene is an alkane obtained from fractional distillation of
is an alkane obtained from fractional distillation of
crude petroleum. Write an equation to show that kerosene can be usedas a source of energy.
2.7. Uses of alkanes
2. Reaction with halogens (halogenations)
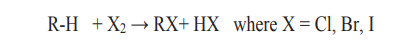
Example: Reaction of methane with bromine
Mechanism of the reaction: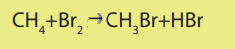
A mechanism of a reaction is a description of the course of the reaction which showssteps of the reaction and the chemical species involved in each step.
The mechanism for the reaction between methane and bromine is the following.i. Phase 1: Initiation (radical formation: formation of Br atom)
ii. Phase 3: Termination steps (Radicals combination and end of the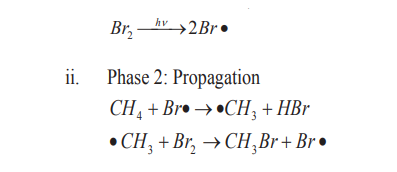
formation of radicals).
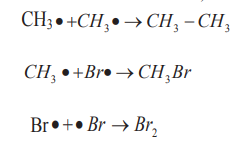
Hence, the generalized reaction

Activity 2.7.
In Rwanda, gas methane has been discovered in Lake kivu and the government
is under its exploitation.
1. Outline all possible uses of methane gas2. Discuss the economic impact of the gas to the livelyhood of Rwandans.

Picture 2.1: Kivu watt power station
3. With the help of the pictures below, deduce the uses of alkanes
1. Methane
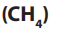
Methane finds many uses:
• It is used as a fuel at homes, ovens, water heaters, kilns and automobiles
as it combusts with oxygen to produce heat.
• Highly refined liquid methane is used as rocket fuel.
• Methane is used as fuel for electricity generation.
• It is used as a vehicle fuel in the form of liquefied natural gas (LNG).
• Methane can be used as raw material in the production of urea, afertilizer.
In general, methane is more environmental friendly than gasoline/petrol and diesel.
2. Butane
• Butane is a key ingredient of synthetic rubber.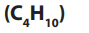
• It is used as fuel in cigarette lighters.
• When blended with propane and other hydrocarbons, it may be referred
to commercially as LPG, for liquefied petroleum gas.
• Butane gas cylinders are used in cooking.• Also used in aerosol spray cans.
3. Propane
• Propane is used as a propellant for aerosol sprays such as shaving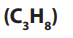
creams and air fresheners.Used as fuel for home heat and back up
electrical generation in sparsely populated areas that do not have
natural gas pipelines.
• Propane is commonly used in movies for explosions
4. Ethane
• Ethane is used in the preparation of ethene and certain heavier
hydrocarbons.
• Ethane can be used as a refrigerant in cryogenic refrigeration systems.
5. Pentane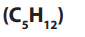
• Pentane is used in the production of polystyrene foams and other foams.
• Used in laboratories as solvents.
• It is also an active ingredients of pesticides.
• Used as solvent in liquid chromatography
6. Hexane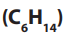
• It is used in the formulation of glues for shoes, leather products, and
roofing.
• It is also used to extract cooking oils such as canola oil or soy oil from
seeds.
• Hexane is used in extraction of pyrethrine from pyrethrum; e.g. Horizon
SOPYRWA (a pyrethrum factory in Musanze District).
• Also for cleansing and degreasing a variety of items, and in textilemanufacturing.
7. Heptane
• Heptane is used as solvent in paints and coatings.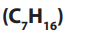
• Pure n-heptane is used for research, development and pharmaceutical
manufacturing.
• Also as a minor component of gasoline.• It is used in laboratories as a non-polar solvent.
2.8. End Unit Assessment
1. Give the general formula of alkanes
2. Answer by True or False
a. 2,2-dimethylbutane is an isomer of hexane
b. Boiling point of alkanes increases with increasing the length of the
chain. Explain why?c. Alkanes are polar molecules; justify your answer
3. Draw the structures of the following formulas:
a. 2, 3, 5-trimethyl-4-propylheptane
b. 2, 2-dimethylpropane
c. 2-methyl pentaned. 4-ethyl-2, 3-dimethyloctane
4. Explain the different steps of the chlorination reaction of methane
5. An alkane with molecular mass of 72 forms only one monochlorinated
product. Suggest the structure of the alkane.
6. a) What do you understand by the term hydrocarbon?
b) What is the relationship between the number of carbon atoms in ahydrocarbon and its boiling point?
c) The hydrocarbon C5H12 burns to form carbon dioxide and water.Write the balanced equation for the reaction.
d) Name the environmental problem that is caused by the formation ofcarbon dioxide during the combustion of hydrocarbon.
7. Consider the alkane with the formula CH3-CH2-CH2-CH2-CH2-CH3
a. Determine the percentage composition of carbon and hydrogen in
the compound,
b. Determine the empirical formula of the above compound,
c. From the results in a) calculate the molecular formula of the
compound,
d. Write down the balanced chemical equation of combustion of the
compound,
e. Name the environmental problem that is caused by the performance of
the reaction in d) and suggest different ways to solve that environmental
problem.
8. Show how each of the following conversions can be accomplished with agood yield
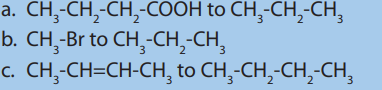
9. a. Referring to methane as an example of alkanes, discuss the importance of
alkanes in our every day life.
b. Gaz methane is extracted in Lake Kivu in Rwanda. Explain its perspectivesin Rwandan economy?
UNIT 3: ALKENES AND ALKYNES
Key unit competency
Relate the physical and chemical properties of alkenes and alkynes to their reactivity and uses.
Learning objectives
• Explain the reactivity of alkenes in comparison to alkanes
• Explain the existence of geometrical isomerism in alkenes
• Describe the industrial process of preparing alkenes and alkynes
• Apply IUPAC rules to name alkenes and alkynes
• Carry out an experiment to prepare and test ethene gas
• Outline the mechanisms for electrophilic addition reactions for alkenes and
alkynes
• Write the structural formulae of straight chain alkenes and alkynes
• Apply Markovnikov’s rule to predict the product of hydrohalogenation of
alkenes
• Classify alkynes as terminal and non-terminal alkynes using their different
structures
• Appreciate the combustion reaction as source of fuels.
• Appreciate the uses and dangers of addition polymers (polythene used forpolythene bags, polypropene for plastic bottles etc.)
Introductory ActivityObserve the following picture and answer the questions that follow.
1. What is the collective name of the substances used to manufacture the
items showed in the above picture?
2. a). What are the raw materials used in the manufacture of the
substances identified in 1)?
b). These raw materials may be obtained from different sources. Discuss
this statement.
c). Do you expect these raw materials be soluble or not in water? Justify
your answer.
3. Even though the items which appear in the picture above are interesting,they also present some disadvantages. Discuss this statement.
3.1. Definition, structure and nomenclature of alkenes
Activity 3.1.1
1. Describe the formation of a carbon-carbon double bond. What is the
hybridisation state of a carbon doubly bonded?2. What is the shape of the molecule around the double bond? Explain.
Alkenes are a homologous series of hydrocarbons which contain a carbon-carbon
double bond. Since their skeleton can add more hydrogen atoms, they are referredas unsaturated hydrocarbons.
The general formula of alkenes is

Example: Ethene
Alkenes are abundant in the nature and play important roles in biology. Ethene,
for example, is a plant hormone, a compound that controls the plant’s growth and
other changes in its tissues.
Ethene affects seed germination, flower maturation, and fruit ripening.
They are described as unsaturated hydrocarbons because they can undergoaddition reactions.
The double bond in alkenes is made of one sigma bond and one pi bond. This gives
rise to the resistance of rotation around the double bond.
The hybridization state in alkenes is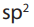
and the structure around each carbon doubly bonded is trigonal
planar with a bond angle value of
Activity 3.1.2
Refer to the IUPAC system used in the nomenclature of alkanes, name thefollowing compounds.
IUPAC names of alkenes are based on the longest continuous chain of carbon atoms
that contains the double bond.
The name given to the chain is obtained from the name of the corresponding alkane
by changing the suffix from –ane to –ene.
If the double bond is equidistant from each end, number the first substituent that
has the lowest number. If there is more than one double bond in an alkene, all of
the bonds should be numbered in the name of the molecule, even terminal double
bonds. The numbers should go from lowest to highest, and be separated from one
another by a comma.
The chain is always numbered from the end that gives the smallest number for the
location of the double bond.
In naming cycloalkenes, the carbon atoms of the double bond are numbered 1 and 2
in the direction that gives the smallest numbers for the location of the substituents.
If a compound contains two or more double bonds, its location is identified by a
prefix number. The ending is modified to show the number of double bonds:
• a diene for two double bonds,
• a triene for two three bonds• a tetraene for four double bonds
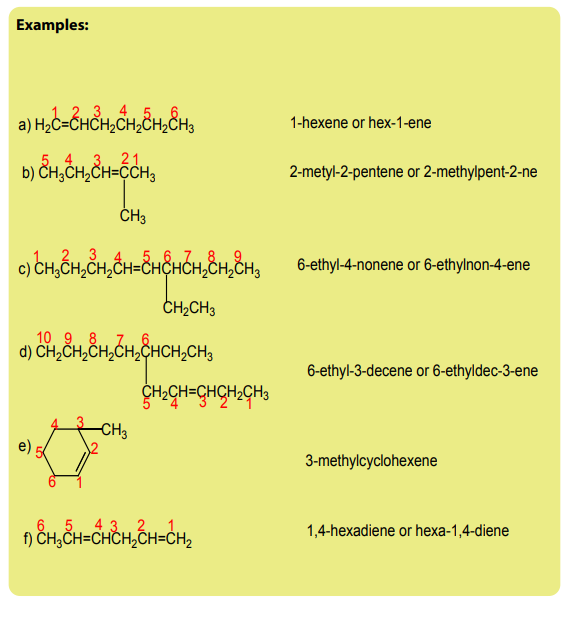
Checking up 3.1
1. Write the structural formula of:
a. 4-ethylhept-3-ene
b. 5-isopropyl-2,6-dimethylundec-3-ene
c. 3-ethyl-2,4,5-trimethyl oct-2-ene
d. 3-ethyl-2-methylcyclohexene
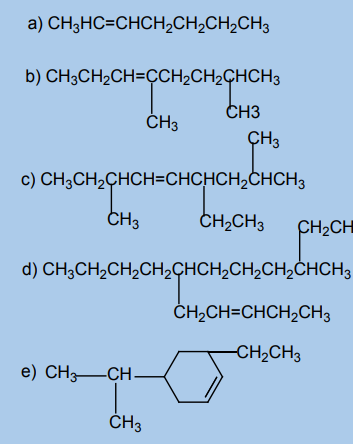
e. Buta-1,2-adiene2. Name each of the following compounds according to the IUPAC system.
3.2. Isomerism in alkenes
Activity 3.2
1. What is meant by isomers and what are the types of isomers?2. Which types of isomerism can be exhibited by alkenes? Give your reasons
Alkenes exhibit two types of isomerism: structural isomerisms and stereoisomerism.
1. Structural isomerism
Alkenes exhibit position isomerism, chain isomerism and functional isomerism.
In position isomerism, the position of the double bond changes but the length ofthe chain remains the same.
Example:
Alkenes and cycloalkanes have the same molecular formula because they both have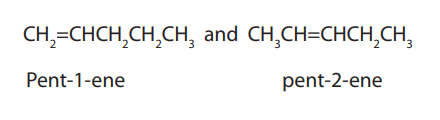
two fewer hydrogen atoms than alkanes. That is why, they have the same molecular
formula. However, they belong to different homologous series. Therefore, they are
functional group isomers. This isomerism that relates open chain compounds toring chain compounds is referred to as ring isomerism.

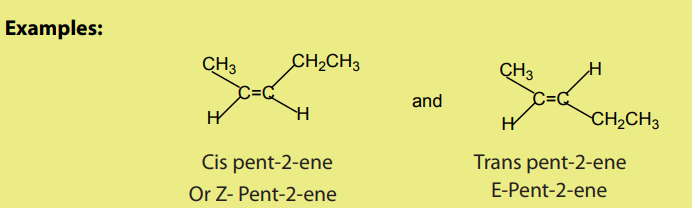
2. Stereoisomerism
Due to the resistance of rotation around the double bond, alkenes give rise to cistransor geometrical isomerism. Sometimes known as E- and Z- Isomers
Checking up 3.2
1. State the necessary condition for the existence of cis-trans isomerism in
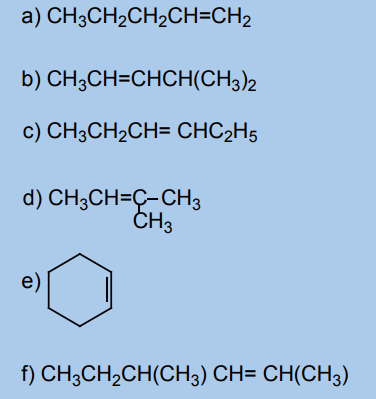
alkenes?2. Which of the following alkenes can exhibit a cis-trans isomerism?
3.3. Preparation of alkenes
Activity 3.3
Different methods can be used to prepare alkenes. Discuss the possible
reactions which may be involved in the preparation of alkenes and propose themechanisms, where it is possible.
Different methods are used for the preparation of alkenes. Most of them areelimination reactions.
An alkene may be obtained by dehydration of an alcohol. The reaction involves1. Dehydration of alcohols
the loss of H and OH from adjacent carbons of an alcohol to form an alkene. The
dehydration is carried out by heating an alcohol with concentrated sulphuric acid
or 85% phosphoric acid.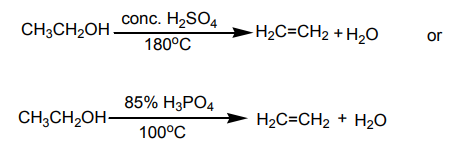
Mechanism of the reaction
The dehydration of alcohols giving alkenes occurs in three steps.
If two or more alkenes may be obtained, the one having more substituents on the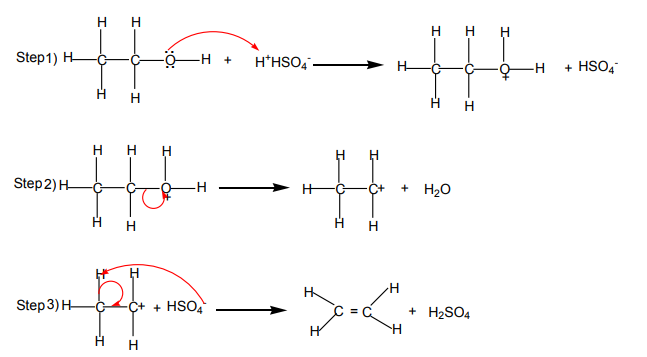
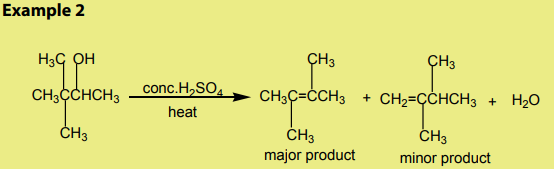
double bond generally predominates. This is the Zaitsev’s rule.This is due to the stability of the intermediate carbocation. The carbocation
produced in step 2 may undergo a transposition (rearrangement) of a hydride ion
or a methyl group giving a more stable carbocation and therefore a more stable
alkene.From the secondary carbocation, two products can be obtained and the reaction
Mechanism
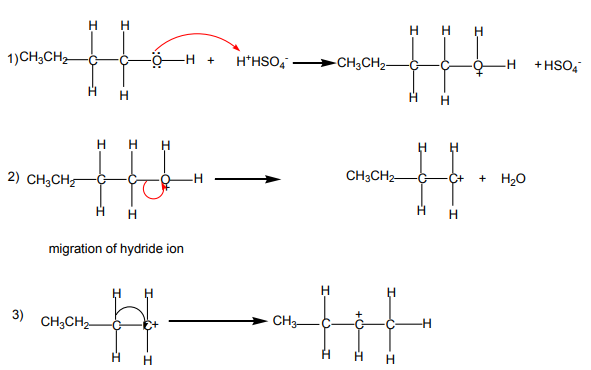
follows the Zaitsev’s rule.
From the tertiary carbocation, two products can be obtained and the reaction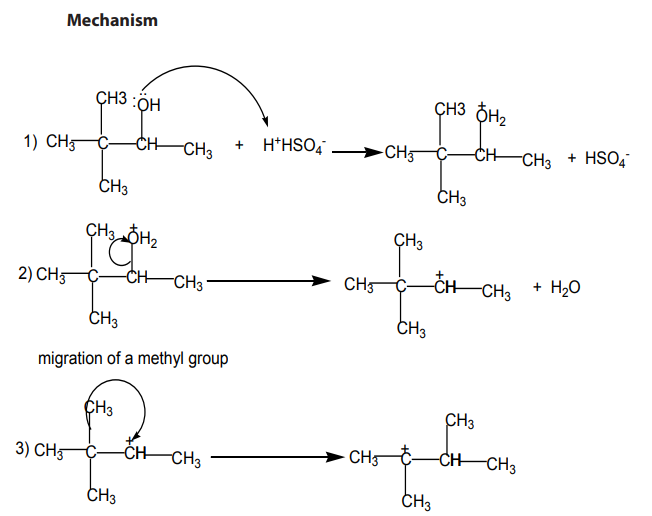
follows the Zaitsev’s rule.
The dehydration of alcohols leading to alkenes may also be effected by heatingalcohols in the presence of alumina.
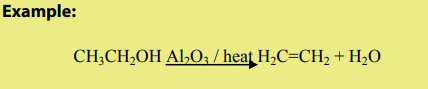
2. Dehydrohalogenation of halogenoalkanes
Halogenoalkanes react with hydroxide ions in ethanolic solution to yield alkenes.The reaction follows the Zaitsev’s rule.
Examples
When a compound containing two halogen atoms on the adjacent carbon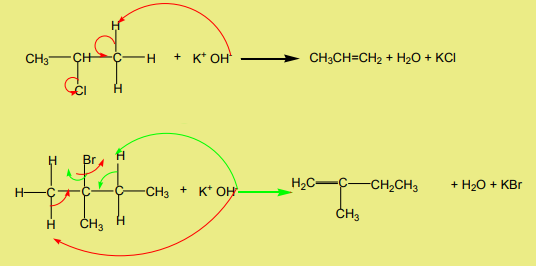
3. Dehalogenation of dihalogenoalkanes
atoms is treated with magnesium or zinc it transforms to an alkene.
Examples:
When the two halogen atoms are attached to non-adjacent carbon atoms, a cyclic
alkane is formed.

Checking Up 3.3
1. Refer to the IUPAC system, name the alkenes formed when the following
alcohols are dehydrated in the presence of sulphuric acid.
a. Pentan-2-ol
b. 2-methylpropan-1-ol
c. 2,3-dimethylbutan-2-ol
d. 2-methylcyclohexanol
e. 2-methylbutan-2-ol
2. What are the products of the dehydrohalogenation of the following
compounds? Show the major product.
f. 1-bromo-2-methylpropane
a. 2-bromo-3-methylpentane
b. 2-bromo-2,3-dimethylbutanec. 3-chloro-3-ethylpentane
3. Write the formula of the compounds formed when each of the followingdihalogenoalkanes react with magnesium.
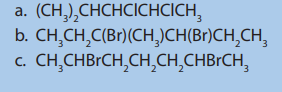
3.4. Laboratory preparation and chemical test for ethene
Activity 3.4
Preparation of ethene Set up the apparatus as shown in the Figure below (Figure
3.1) and follow the instructions to perform the experiment on the preparation ofethene.
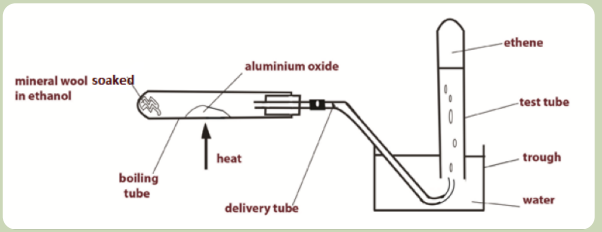
Figure 3.1: Laboratory preparation of ethene
Requirements:
Chemicals:
• Ethanol, aluminium oxide, lime water, mineral wool, bromine water, acidified
potassium permanganate solution (very dilute), water.
Additional apparatus:
• Boiling tube
• Rubber stopper with hole
• Delivery tube
• Trough
• Test- tube rack
• 5 test tubes
• 5 rubber stoppers for test tubes
• Spatula Procedure and setting
• Bunsen burner
• Glass rod
• Splint• Matches
1. Preparation of ethene:
- Pour some ethanol into the boiling tube to a 3 cm depth
- Add some glass wool to soak up the ethanol, using a glass rod to push
the wool down the tube.
- Clamp the boiling tube in a horizontal position using a retort stand.
- Put a small amount of aluminium oxide about half way along the
boiling tube. - Complete the set up of the apparatus as shown in the
diagram above.
- Light the Bunsen burner, adjust it to a blue flame and heat the
aluminium oxide. (Make sure the test tube is filled with water when
you start to collect the gas produced.)
- As the aluminium oxide gets hot the heat reaches the ethanol at the
end of the tube. The ethanol then changes to vapour, passes over the
hot aluminium oxide and is dehydrated to produce ethene gas.
- Collect 5 test tubes of the gas and put a stopper on each tube when
it is filled. - When the test tubes have all been filled, loosen the retort
stand and raise the apparatus so that the delivery tube no longer dips
into the water. This avoids suck back of water as the tube begins to
cool which could cause the boiling tube to crack. Turn off the Bunsen
burner.
2. Testing the properties of ethene
Addition of bromine:
- Taking great care, add about 1ml of the test tube of bromine water to
one of the test tubes of ethene.
- Replace the stopper and shake the tube a few times.
- Record your observations.
- Write down your conclusions
- Addition of acidified potassium permanganate:
- Add about 1ml of very dilute potassium permanganate solution to
one of the test tubes of ethene and shake the tube a few times.
- Record your observations.
- Write down your conclusions
Combustion:
- Remove the stopper of one of the tubes filled with ethene and apply a
light to the mouth of the test tube using a lighted splint.
- Allow the gas to burn and when it has stopped burning add a small
amount of lime water to the test tube, stopper it and shake the tube
a few times.- Write down your observations.
Interpretation
When ethanol is heated in the presence of aluminium oxide, a gas is produced. This
gas does not react with lime water. This means that the produced gas is not carbondioxide. The equation of the reaction is:
The gas decolourises bromine water. Bromine water is a test used to identify the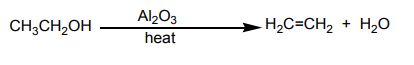
presence of a carbon-carbon double bond or triple bond. The bromine adds across
the double bond and a dibromoalkane is formed. The reaction between alkene andbromine water is shown below:
If you shake an alkene with bromine water (or bubble a gaseous alkene through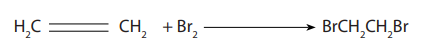
bromine water), the solution becomes colourless. Alkenes decolourise bromine
water.
The Figure 3.2 shows Bromine water added to ethene: before the reaction (left)
the color of bromine appears, and after the reaction (right) the colour of brominedisappears.

Picture 3.1: Test for unsaturation
When ethene reacts with acidified potassium manganate (VII), the purple colour of
the permanganate solution turned to colourless or light pink indicating the presenceof the carbon – carbon double bond.The reaction is the following:
The gas burns with a smoky flame producing carbon dioxide and heat energy. The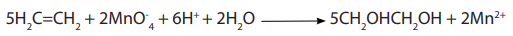
carbon dioxide produced turns into milky lime water.
Checking Up 3.4
1. Ethene is prepared by dehydration of ethanol in the presence of alumina,
explain other reactions that produce ethene.
2. Describe the chemical test used to identify the presence of a carbon-carbon
double bond in an organic compound.
3. Explain how ethene can be differentiated from carbon dioxide using achemical test?
3.5. Physical properties of alkenes
Activity 3.5
1. How does the physical state of alkenes change with molecular mass.
2. Put in a test tube 5ml of cyclohexene. Add 5ml of water and mix. Record
your observations.
3. Put in a test tube 5ml of cyclohexene. Add 5ml of tetrachloromethane
and mix. Record your observations.
4. Cis-but-1-ene and trans-but-2-ene exhibit geometric isomerism. Statewhich one of them is less volatile and why?
• Alkenes which have less than 5 carbon atoms are gaseous at ordinary
temperature, the other are liquid up to 18 while others are solids as the number
of carbon atoms increases.
• Boiling points and melting points of alkenes are less than those of alkanes but
also increase as the molecular weight increase.
• Alkenes are insoluble in water but soluble in most organic solvents.
• Cis-alkenes have a slightly higher boiling point than the trans-isomers becausethe dipole moments in trans structures cancel each others----.
Checking Up 3.5
Which one of the following compounds has higher boiling point? Explain.
a. Cis-butene and trans-butene
b. Ethene and propene
3.6. Chemical properties
3.6.1. Addition reactions3.6.1.1. Electrophilic additions
Activity 3.6.1
1. Explain the following terms and give two examples for each.
a). Addition reaction
b). Lewis acid
2. Distinguish other name given to a Lewis acid.
3. Predict if Lewis acids can react with alkanes.
4. Justify if Lewis acids react with alkenes.5. Differentiate the reactivity of alkenes and alkanes.
Alkenes are far more reactive than alkanes due to the carbon-carbon double bond.
These compounds are unsaturated and they can easily undergo addition reactions
to yield saturated products.
The double bond in alkenes is a region of high density of electrons. Therefore, this
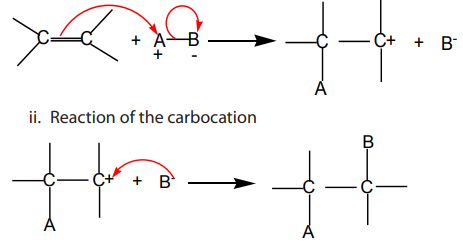
region is readily attacked by electrophiles. An electrophile is an atom, a molecule oran ion which is electron-deficient; i.e. it is a Lewis acid or an electron pair acceptor.
Electrophilic addition reactions take place in two steps:
i. Formation of a carbocation
1. Addition of hydrogen halides
Hydrogen halides (HCl, HBr, HI) react with alkenes to yield halogenoalkanes. The
reaction is carried out either with reagents in the gaseous state or in inert solventsuch as tetrachloromathane.

When hydrogen halides add to unsymmetrical alkenes, the reaction leads to the
formation of two products in two steps. The first step leads to the formation oftwo different carbocations with the major product formed from the more stable
carbocation. This is the Markownikov’s rule. That is “The electrophilic addition of an
unsymmetric reagent to an unsymmetric double bond proceeds by involving the moststable carbocation.
The order of stability of the carbocations is:

In the presence of peroxide, the reaction follows a free radical mechanism and it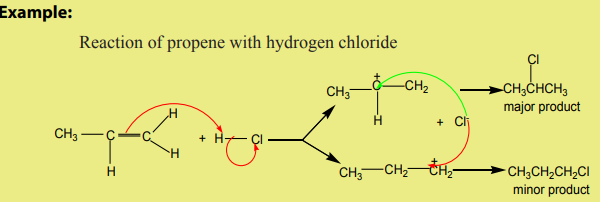
does not follow the Markonikov’s rule.


2. Addition of water
The hydration of alkenes catalysed by an acid is an electrophilic addition. Ethene
can be transformed into ethanol. The first step consists of adding concentrated
sulphuric acid. The second step consists of the hydrolysis of the product of the
first step.
In industry the reaction is carried out at approximately 300 °C in the prence ofphosphoric acid as a catalyst.
3. Addition of cold concentrated sulphuric acid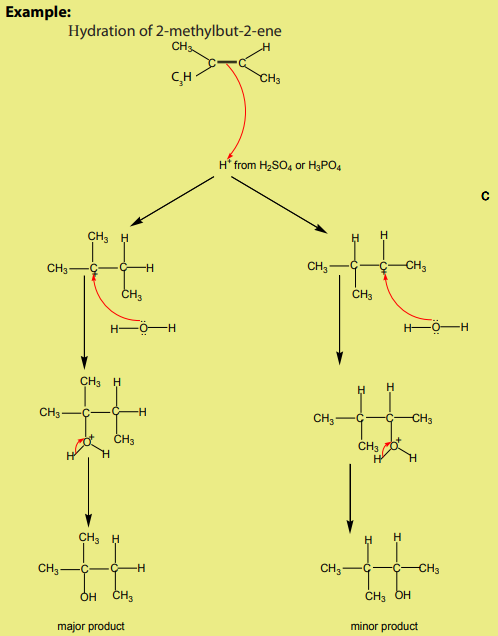
When cold concentrated sulphuric acid reacts with alkene, an alkyl hydrogen
sulphate is obtained. If the starting alkene is unsymmetrical, two different alkyl
hydrogen sulphates are obtained. If the alkyl hydrogen sulphate is warmed in thepresence of water, an alcohol is obtained.

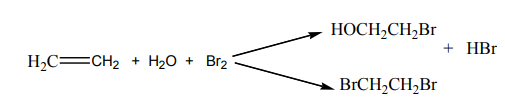
4. Addition of halogens
The addition of halogens (halogenation) on alkenes yields vicinal
dihalogenoalkanes. The reaction takes place with pure reagents or by mixing
reagents in an inert organic solvent.
When a chlorine or bromine molecule approaches an alkene, the pi electronscloud interact with the halogen molecule causing its polarisation.
Example:
Reaction of ethene with bromine in an inert organic solvent gives:

The reaction follows the mechanism below:
he reaction with bromine is a useful test for alkenes.
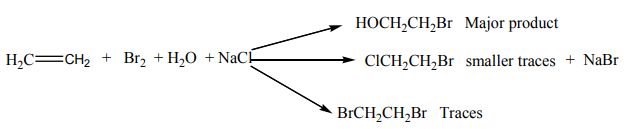
The brown red colour of bromine is discharged in alkenes.With bromine water, the reaction gives a mixture of organic products.
Example:
Bromine water containing sodium chloride gives a mixture of three organic products.
Example:
3.6.1.2. Hydrogenation
In the presence of a catalyst (Pt, Ni, Pd), alkenes react with hydrogen to give alkanes.
This reaction is very useful when transforming vegetable oils into fats such as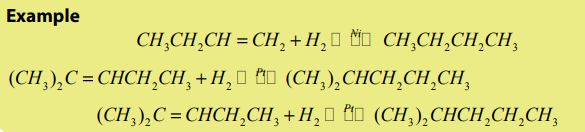
margarine by hydrogenation. The process is referred as hardening.
Checking up 3.6.1
1. Predict the products formed when alkenes (But-1-ene and
3-methylpent-2-ene react with each of the following reactants:
i. HCl
ii. Water in acidic medium
iii. Cold sulphuric acid
iv. hydrogen
2. Outline the mechanism of the reaction between 2-methylpent-2-enewith hydrogen bromide.
3.6.2. Oxidation reactions
Activity 3.6.2
1. Explain the terms oxidation, oxidising agent based on examples
2. Explain the terms reduction, reducing agent and give examples
3. The combustion of alkenes yields products, illustrate it by a reaction and
indicate the types of products generated.
4. Explain what happens when alkenes react with oxidising agents.
Alkenes are readily oxidised due to the presence of the double bond.
1. Reaction with oxygen
i. Transformation to epoxidesEthene react with oxygen in the presence of silver as a catalyst to yield epoxyethane.
Epoxyethane is a very reactive substance. It reacts with water to give 1,2-ethanediol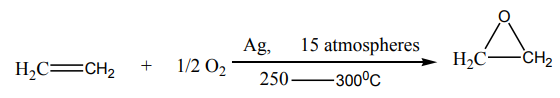
which is used in the making of polyesters, detergents, and so on.
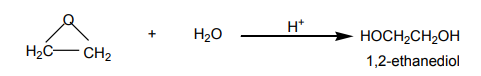
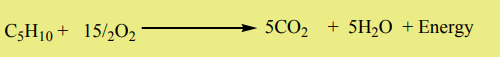
ii. Combustion
Alkenes burn in oxygen to give carbon dioxide, water and energy

Example:
2. Reaction with ozone
An alkene reacts with ozone to give an ozonide.
The reaction is carried out at low temperature (below ) in non-aquous medium.
) in non-aquous medium.
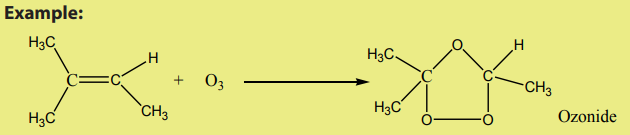
On hydrolysis, the ozonide splits into two carbonyl compounds. The reaction which
is an oxidative cleavage is referred to as ozonolysis.
Since the by-product is hydrogen peroxide, the hydrolysis is carried out in thepresence of a reducing agent.

The interest of the ozonolysis reaction is that it can help to identify the location of
the double bond in an alkene.
3. Reaction with potassium permanganate
Alkenes react with dilute potassium permanganate solution to give diols. The
reaction takes place in the cold.The colour change depends on the medium of the reaction.
This reaction also is used to test for the presence a double bond.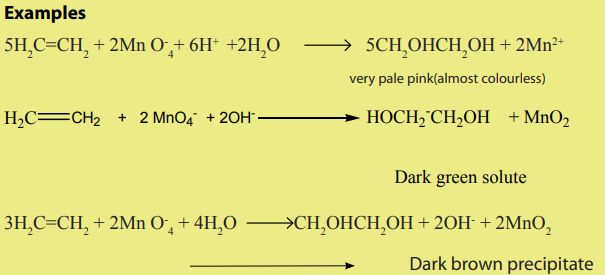
An alkane does not react with KMnO4 (left), but an alkene reacts with KMnO4producing a dark brown precipitate of MnO2 (right) (Figure 3.3).

Picture 3.2: Reaction of alkenes and KMnO
4. Hydroformylation
The hydroformylation is a process by which alkenes react with carbon monoxideand hydrogen in the presence of rhodium catalyst to give aldehydes.
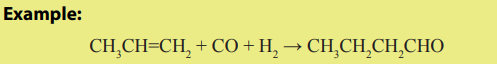
Checking up 3.6.2
1. Write the equations of the reaction between 3-methylpent-2-ene with:
a. Oxygen in the presence of silver catalyst.
b. Cold dilute potassium permanganate solution
c. Ozone
2. Describe the purpose of the reaction between alkenes and ozone.
3. Describe the observations when butane and but-2-ene react separatelywith potassium manganate (VII) solution.
3.6.3. Addition polymerisation
Activity 3.6.3
1. The students of a given class are asked to form separate couples of students.
In each couple, the students hold each other by their two hands. Now
each couple is asked to free one hand per student so that each student of
each couple can hold a hand of another student from a different couple.
What will be the result of such an arrangement compared to the first one?
2. From this example, predict what will happen in an addition reaction ofmany molecules of one or different alkenes?
Alkenes undergo addition polymerisation reaction to form long chain polymers.i.e
a polymer is a large molecule containing a repeating unit derived from small unit
called monomers. A polymerisation reaction involves joining together a large
number of small molecules to form a large molecule.
Many different addition polymers can be made from substituted ethene compounds.
Each polymer has its physical properties and therefore many polymers have wide
range of uses.Mechanism for the polymerisation of ethene.
1.Initiation
It is a free radical initiation.

2. Propagation
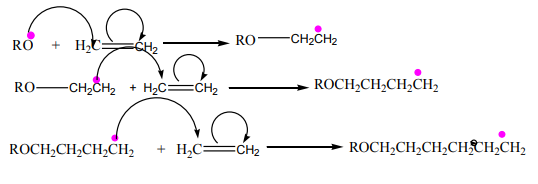
3. Termination
where the part between brackets indicates a unit of the formula of the polymer that
repeats itself in the formula; n indicates the number of the units in a formula of apolymer and is a very large number.
Summary of most alkene polymers obtained from alkenes as monomers and theiruses (Table 3.1)
Table 3.1: Polymers of alkenes and their uses

Checking Up 3.6.3
1. Explain the terms
a. addition polymerisation
b. monomer
c. polymer
2. The use of some plastic bags is banned in our country. Analyse the
scientific and environmental reasons of this prohibition and suggestalternative solutions.
Project Work
Although they have many uses, plastics have side effects and therefore some of
them are being replaced by more eco-friendly plastics.
Design a project for the making of plastics using starch from plants. In your
project you will:
1. Perform the extraction of starch
2. Make plastics using starch you will have extracted
3. Test the properties of your plastics4. Differentiate between bioplastics and biodegradable plastics.
3.7. Structure, classification and nomenclature of alkynes
Activity 3.7
1. Explain the formation of a carbon-carbon triple bond.
2. What is the hybridisation state of a carbon atom triply bonded and what
is the shape of the structure around it.3. Differentiate between the following compounds
4.
A triple bond consists of one sigma bond and two pi bonds. Each carbon of the triple
bond uses two sp orbital to form sigma bonds with other atoms. The unhybridised 2p
orbitals which are perpendicular to the axes of the two sp orbitals overlap sidewaysto form pi bonds.
According to the VSEPR model, the molecular geometry in alkynes include bond
angle of 180o
around each carbon triply bonded.Thus, the shape around the triplebond is linear.
There are two types of alkynes: terminal alkynes and non-terminal (internal) alkynes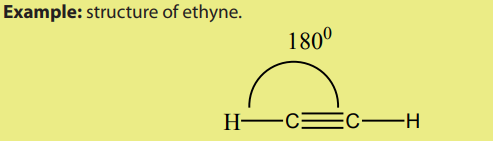
A terminal alkyne has a triple bond at the end of the chain e.g.: :
A non-terminal alkyne has a triple bond in the middle of the chain:

Examples:
Alkynes are named by identifying the longest continuous chain containing the triple
bond and changing the ending –ane from the corresponding alkane to –yne.

Checking Up 3.7
1. Name according to the IUPAC system, each of the following compounds.
5. Write structural formula for:
a. 2,5- dimethyl-3-hexyne
b. 6-isopropyl-5-propyldec-3-ynec. 5-ethyl-4-methloct-1-yne
3.8. Laboratory and industrial preparation of alkynes
1. Preparation of ethyne
Activity: 3.8Set up the apparatus as shown in the diagram below

Figure 3.3 Laboratory preparation of ethyne
Procedure:
• Place 2g of calcium carbide in a conical flask
• Using the dropping funnel, add water drop by drop.
• Collect the gas produced in the test tube.
• Remove the first tube and connect a second test tube.
• To the first test tube add two drops of bromine water. Record your
observations
• To the second tube add two drops of potassium manganate (VII). Record your observations.
Ethyne (acetylene) can be prepared from calcium carbide which is obtained byreduction of calcium oxide by coke at high temperature.
A more quick industrial production consists of heating methane alone at high
temperature for 0.01-0.05second.
When bromine water is added to acetylene, the red colour of bromine is discharged.
The solution becomes colourless. The decolourisation of bromine water is a test
for unsaturation in a compound.
When potassium manganate (VII) is added to acetylene, its purple colour isdischarged.
2. Alkylation of acetylene
The hydrogen atom of ethyne as that of other terminal alkynes is slightly acidic and
therefore it can be removed by a strong base like NaNH2 or KNH2
.The products of the reaction are acetylides. Acetylides react with halogenoalkanes
to yield higher alkynes.
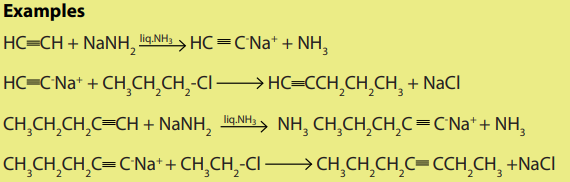
3. Dehydrohalogenation
The dehydrohalogenation of vicinal or geminal dihalogenoalkanes yields alkynes


4. Dehalogenation
The dehalogenation of a tetrahalogenoalkane yield an alkyne.

Checking Up 3.8
1. Using chemical equations, describe the preparation of ethyne(acetylene)
2. By which reactions higher members of the alkynes family are prepared?
3. Suggest a synthesis for each of the following compounds using
acetylene as the starting organic material.
a. Propyne
b. 2-butynec. 3-hexyne
3.9. Physical properties of alkynes
Activity 3.9
Alkynes have the general formula
They have two fewer hydrogen atoms
than alkenes, and four fewer H than alkanes. Do you expect alkynes to be more
or less volatile than alkenes? Explain by referring to the nature of the chemicalbonding and the structure of alkenes and alkynes.
Alkynes are non-polar compounds with physical properties similar to those of
alkenes with the same number of carbon atoms. Their linear structure gives themgreater intermolecular forces than alkenes

Alkynes are water insoluble but they dissolve in each other and in non-polar solvents.
Checking up 3.9
1. Which of 3,4,4-trimethylpent-1-yne and oct-3-yne has a high volatility?
Explain2. Table salt (NaCl) is water soluble but hex-2-yne is not. Explain why.
3.10. Chemical reactions of alkynes
Activity 3.10
Alkynes have a carbon-carbon triple bond. That is why they have a higher electron
density than alkenes. Do you expect alkynes to be more reactive than alkenes?Which types of reactions can be exhibited by alkynes?
Addition reactions
As unsaturated hydrocarbons, alkynes are very reactive. Because they are unsaturated
hydrocarbons, alkynes undergo addition reactions. Alkynes can add two moles of
reagents.
Even though they have a higher electron density than alkenes, they are in general
less reactive because the triple bond is shorter and therefore the electron cloud is
less accessible.
1. Addition of hydrogen halides
Alkynes react with hydrogen halides to yield vicinal dihalogenoalkanes, thereaction follows the Markownikov’s rule. The reaction takes place in four steps.
Example:
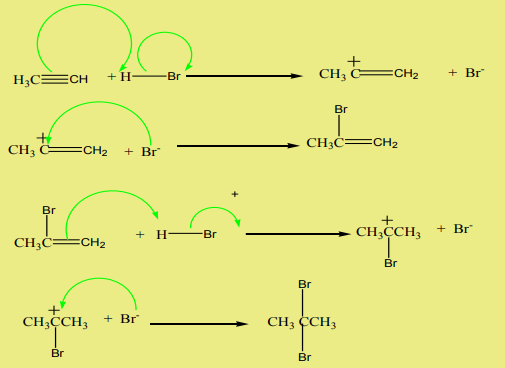
2. Addition of water
Alkynes react with water in the presence of sulphuric acid and mercury sulphate
at to give carbonyl compounds.
to give carbonyl compounds.
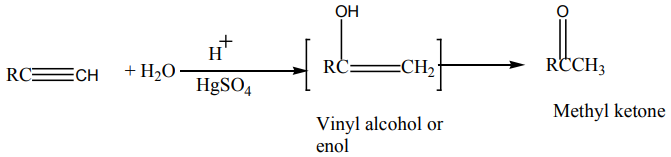
Example: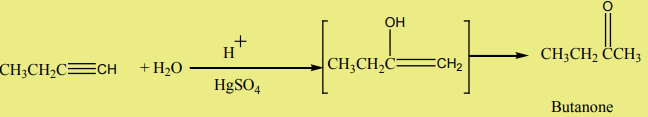
3. Hydrogenation
The hydrogenation of alkynes in the presence of palladium catalyst gives alkanesThe reaction requires two moles of hydrogen for a complete saturation.
Example:
In the presence of Lindlar catalyst, the alkynes are partially hydrogenated giving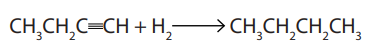
alkenes
A Lindlar catalyst is a heterogeneous catalyst that consists of palladium deposited
on calcium carbonate and poisoned with different lead derivatives such as lead
oxide or lead acetate.
Reaction with metals
Terminal alkynes react with active metals to yield alkynides and hydrogen gas.Internal alkynes do not react as they do not have an acidic hydrogen atom.

4. Reaction with metal salts
When a terminal alkyne is passed through a solution of ammoniacal silver nitrate, awhite precipitate of silver carbide is formed.
When a terminal alkyne is passed through a solution of ammoniacal copper(I)
chloride, a red precipitate of copper(I)carbide is formed.
The reactions above are used to:
• Differentiate between terminal and non-terminal alkynes.
• Differentiate ethene and ethyne
The reaction shows that hydrogen atoms of ethyne are slightly acidic, unlike thoseof ethene.
Checking up 3.10
1. Write the formula(s) and the name (s) of the products of the reaction of
pent-1-yne with:
a. water
b. hydrogen chloride
c. sodium metal
2. Outline the mechanism of the reaction between but-2-yne with
hydrogen bromide.
3.11. Uses of alkenes and alkynes
Activity 3.11
Look at the picture below and mention the importance of alkenes and alkynes.
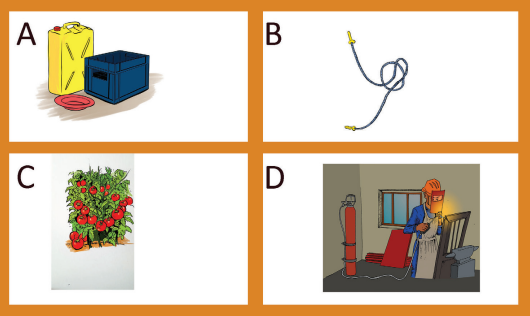
Figure 3.5: Some plastic materials (A& B), tomatoes which are ripening (C)
and a person who is welding (D)
• Alkenes are extremely important in the manufacture of plastics which have
many applications such as: packaging, wrapping, clothing, making clothes,
artificial flowers, pipes, cups, windows, ...
• Ethene is a plant hormone involved in the ripening of fruits, seedgermination, bud opening;
Picture 3.3: Ethene is a plant hormone which causes bananas to ripen.
image source m.yukie .mobi.
• Ethene derivatives are also used in the making of polymers such as
polyvinylchloride (PVC), Teflon,...
• Alkenes are used as raw materials in industry for the manufacture of
alcohols, aldehydes, ...
• Alkynes are used in the preparation of many other compounds. For example
ethyne is used in the making of ethanal, ethanoic acid, vinyl chloride,
trichloroethane, ...
• Ethyne (acetylene) is used as a fuel in welding and cutting metals.• Propyne is used as substitute for acetylene as fuel for welding.
Checking up 3.11
Alkenes, alkynes and their derivatives have many applications in our daily life.Discuss this statement.
3.12. End unit assessment
I. Multiple choice questions. Choose the best answer in the following by
noting the corresponding letter.
1. Which of the following is given off during ripening of fruits and
vegetables?
b. Ethane
c. Ethene
d. Ethyne
e. Methane
2. Loss of hydrogen halide is called:
a. Halogenation
b. Dehydration
c. Dehydrohalogenation
d. Hydrogenation
3. Alkenes can be oxidized using powerful oxidizing agent in acidified
medium.
a. Potassium manganate (VII)
b. Sodium manganate (VI)
c. Calcium manganate (VI)
d. All of them
4. The molecular formula of-------- fit the general formula
a. Alkanes
b. Alkynes
c. Alcohols
d. Alkenes
5. Example of addition reactions include all but one of the following.
Which is the odd one out?
a. Combustion of propene.
b. Reaction of with propene.
with propene.
c. Reaction of HBr with but-2-ene.
d. Polymerization of ethene
6. Which statement is incorrect about reactions of propene?
a. Reaction with and
and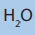 gives 1-bromo propan-2-ol as the main
gives 1-bromo propan-2-ol as the main
product.
b. Polymerization of propene gives polypropene, of which the isotactic and
syndiotactic forms are commercially valuable.
c. Reaction with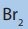 in the presence of a radical initiator yields
in the presence of a radical initiator yields
2-bromopropane as the major product.
d. No correct answer
7. Which one of the following statements is incorrect?
a. The electrophilic addition of HBr to but-2-ene involves a secondary
carbonium ion intermediate.
b. In the presence of a radical initiator, HBr reacts with but-1-ene to give
1-bromobutane as the major product.
c. In the presence of a radical initiator, HBr reacts with but-1-ene to give a
Markovnikov addition product.
d. The major product of the electrophilic addition of HBr to hex-1-ene is
2-bromohexane.
8. What type of reaction do alkynes undergo across triple bond?
a. Elimination reaction
b. Substitution reaction
c. Addition reaction
d. Halogenation
96 Chemistry Senior Five Student Book
9. Acetylene is also called:
a. Ethyne
b. Ethene
c. Ethane
d. Methane
10. What product(s) will be obtained from the acid-catalysed hydration of
pent-2-yne?
a. pentanal
b. pent-2-one and pentan-3-one
c. pentan-2-one
d. pentan-3-one
II. Open questions
11. Give all possible isomers of
12. Explain the following observations
a. When bromine in presence of dichloromethane is added to propene,
only one product is formed i.e. 1,2-dibromopropane.
b. When bromine water is added to propene, a mixture of
is added to propene, a mixture of
products namely 1,2-dibromopropane and bromopropan-2-ol are
obtained.
c. When bromine in presence of carbon tetrachloride and sodium chloride
is added to propene, a mixture of products namely, 1,2-dibromopropaneand bromo-2-chloropropane are formed.
13. Show how the following conversions may be accomplished
14. a. In an experiment it was found that 35g of pure alkene reacted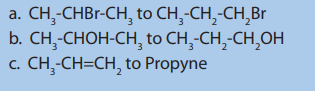
with 100g of bromine .
i. Calculate the molecular mass of the alkene
ii. Write the molecular formula of the alkene
iii. Write the structural formulae and the systematic names of one of
any two alkenes in (ii)
b. Using equations only show the mechanism for the reaction of oneof alkenes in (iii) with bromine.
15. Three hydrocarbons D, E and F, all have the molecular formula C6H12.
D decolourises an aqueous solution of bromine and shows geometric
isomerism. E also decolourises an aqueous solution of bromine but does
not show geometric isomerism. F does not decolourise an aqueous solution
of bromine. Draw one possible structure each for D, E and F.
16. Alkenes such as ethene and propene have been described as the building
blocks of the organic chemical industry. Discuss this statement, giving
examples. What particular features of the chemistry of alkenes make themsuitable for this role and why are alkanes less suitable.
UNIT 4: HALOGENOALKANES (ALKYL HALIDES)
Key unit competency
The learner should be able to relate the physical and chemical
properties of halogenoalkanes to their reactivity and their uses
Learning objectives
• Define halogenoalkanes and homologous series.
• Explain the reactivity of halogenoalkanes.
• Explain the physical properties of halogenoalkanes.
• Describe preparation methods for halogenoalkanes.
• Explain different mechanisms in halogenoalkanes.
• Explain the uses and dangers associated with halogenoalkanes.
• Draw displayed structural formulae of halogenoalkanes and give names using
IUPAC system.
• Classify halogenoalkanes according to developed formula as primary,
secondary and tertiary.
• Write reaction mechanisms of halogenoalkanes as SN1, SN2, E1 and E2.
• Test for the presence of halogenoalkanes in a given sample organic compound.
• Appreciate the uses and dangers of halogenoalkanes in everyday life.• Develop the awareness in protecting the environment.
Introductory activity
Look at the pictures below and answer the following questions.
Record your answers and discuss them.
a. Observe carefully pictures 4.1 and 4.2 and suggest the similarity
between them.
b. Observe carefully pictures 4.1 and 4.2 and suggest the difference
between them.
Substances which are used in the pictures belong to the same homologous series.
They may be obtained from the reaction between alkanes and halogens.
What homologous series do these substances belong to?
4.1. Definition and nomenclature of halogenoalkane
Activity 4.1
1. Look at the following and answer the questions that follow.

Questions:
i. Which structures do represent halogenoalkanes?
ii. What are the similarities between the selected structures?iii. From your answers above deduce the general formula for alkanes.
1. Definition
Halogenoalkanes compounds are compounds in which the halogen atoms like
chlorine, bromine, iodine or fluorine are attached to a hydrocarbon chain. When
the halogen atom is attached to a hydrocarbon chain the compound is called ahalogenoalkane or haloalkane or an alkyl halide.
Halogenoalkanes contain halogen atom(s) attached to the hybridised carbon
hybridised carbon
atom of an alkyl group.
2. Nomenclature of halogenoalkanes
Halogenoalkanes are organic compounds that contain a halogen atom: F, Cl, Br, I.
They are named using the prefixes fluoro-, chloro-, bromo- and iodo-.
Numbers are used if necessary to indicate the position of the halogen atom in themolecule.
If the molecule contains more than one halogen atom of the same kind, the prefixes
di-, tri-, tetra-, etc… are used.
Examples:
Checking up 4.1
1. Name these compounds
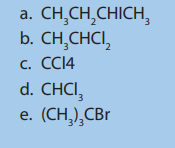
2. Write the structural formulae for the following compounds:
a. 1,2-dibromo-3-chloropropaneb. 1,1,2-trichloro-1,2,2-trifluoroethane
4.2. Classification and isomerism
Activity 4.2
Consider the following compounds and based on the carbon atom attached tothe halogen atom, classify them.
Do research to find an appropriate name for each class
4.2.1. Classification of halogenoalkanesThere are three types of halogenoalkanes:
A primary halogenoalkane has a halogen atom attached to the ended carbon atom
of the chain. A secondary halogenoalkane has a halogen atom attached to a carbon
bonded to two other carbon atoms while a tertiary halogenoalkane has a halogenatom attached to a carbon bonded to three other carbon atoms.
4.2.2. Isomerism
Halogenoalkanes exhibit both chain and position isomerism.
Example: Molecular formula
a. Chain isomerism: This arises due to arrangement of carbon atoms in chains ofdifferent size.
b. Position isomerism: This arises due to the different positions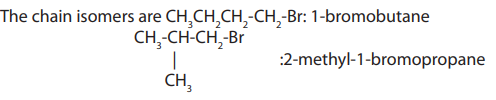
taken by the halogen atom on the same carbon chain.
The following compounds are position isomers: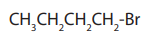 and
and
 because the atoms of bromine are on different positions
because the atoms of bromine are on different positions
of the chain.
Hence, all isomers of the compound with molecular formula
are the following.
Checking Up 4.2:
1. How many positional isomers possess the chlorobromopropane, Enumerate those able to form optical isomers.
Enumerate those able to form optical isomers. Draw their structuralformulae.
2. Illustrate the structural formulas of :a. 1,1,2-trichloropropane
a. 2-chloro-2-methylpropane
4.3. Physical properties of halogenoalkanes
Activity 4.3
1. Consider the following substances:
Sodium chloride, potassium bromide, hexane, pentane, trichlomethane,
terachloromethane.
Mix a sample of each compound (1g for solids, 2ml for liquids) with 10ml
of water.
2. Record your observations.
3. Write down your conclusions.
4. Based on the physical state and the nature of chemical bonding, predict
the increasing order in the boiling points of the compounds above.5. Write down your conclusions.
1. Volatility
Volatility is a property that shows if a substance transforms easily or not into vapour
or gaseous form. This property depends on the nature of the bonds that make up
the molecule of the substance. Generally non polar covalent compounds are more
volatile than polar covalent compounds. We know that halogens when bonded toother atoms form polar bonds because they possess high electronegativities:
F =
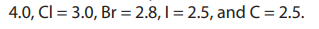
The more the difference of electronegativities of the atoms that form the bond,
the more polar is the bond. This explains the high polarity of C-F bond with an
electronegativity difference of 1.5, and the low polarity of C-Cl and C-Br bonds wherethe electronegativity differences are 0.5 and 0.3 respectively.
The presence of polarity or charge distribution results into more attraction between
polar molecules called dipole-dipole attraction forces, one type of Van der Waalsforces, as shown below:
The dashed line represents the attraction forces between the polar molecules or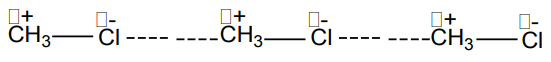
dipoles.
Therefore, more energy must be supplied to separate polar molecules and this
explains why melting and boiling temperatures of fluoroalkanes and chloroalkanes
are higher than those of alkanes of similar molecular mass.
As we have already learnt, molecules of organic halogen compounds are generally
polar. Due to the greater polarity as well as higher molecular mass as compared
to the parent hydrocarbons, the intermolecular forces of attraction (dipole-dipole
and Van der Waals) are stronger in the halogen derivatives. That is why the boiling
points of chlorides, bromides and iodides are considerably higher than those of thehydrocarbons of comparable molecular mass (Table 4.1).
Table 4.1: Comparison of boiling points of some halogenoalkanes
Chloromethane, bromomethane, chloroethane and some chlorofluoromethanes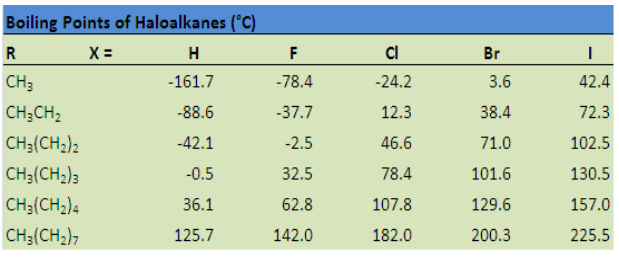
are gases at room temperature. Higher members are liquids or solids.
The attractions get stronger as the molecules get bigger in size. The pattern of
variation of boiling points of different halides is depicted in Figure 4.1. For the same
alkyl group, the boiling points of alkyl halides increase in the order: RF <RCl < RBr, <
RI This is because with the increase in size and mass of halogen atom, the magnitude
of Van der Waal forces increases.
2. Solubility
The solubility is the capacity of a substance to dissolve in a given solvent; in chemistry
the most common solvent we refer to is water. It is a result of the interaction between
the molecules of the substance, a solute, and the molecules of the solvent.
Polar molecules can interact with water molecules, but the attractive forces set
up between water molecules and molecules concerned are not as strong as the
hydrogen bonds present in water. Halogenoalkanes therefore, although they
dissolve more than alkanes, are only slightly soluble in water.
3. State
The state of matter is the physical appearance of that matter: solid, liquid and
gaseous.
Chloromethane, bromomethane, chloroethane and chloroethene are colourless
gases at room temperature and pressure. The higher members are colourless liquids
with a sweet pleasant smell.
4. Density
The density is a measure of the quantity of matter by volume unit. Cotton wool is
less dense than sand because if you compare the quantity of matter cotton wool
and sand contained in for instance you find that there more matter
you find that there more matter
in sand than in cotton wool.
The density of halogenoalkanes increases in the order RCl < RBr < RI, since the
atomic weight of halogens increases in order Cl < Br < I. Iodo, bromo and polychloroderivatives are denser than water but chloro derivatives are less dense than water.
Checking up 4.3
1. Arrange each set of compounds below in order of increasing boiling
points and explain why.
a. Bromomethane, tribromomethane, chloromethane,
dibromomethane.
b. 1-chloropropane, 2-chloro-2-methylpropane, 1-chlorobutane.
2. Explain the origin of the difference between the boiling temperatures ofthe following compounds:

4.4. Preparation methods of halogenoalkanes
1. From alkenes and alkynes
Activity 4.4.1
1. Give the product for each of the following chemical reaction.

2. Identify the class of the products of the reactions above.
Halogenoalkanes can be prepared by a reaction of alkenes or alkynes with:
i. hydrogen halides
Addition of hydrogen halide to alkenes, gives alkyl halides as the products. Theorientation in the addition reaction is described by Markovnikov’s rule (see alkenes).
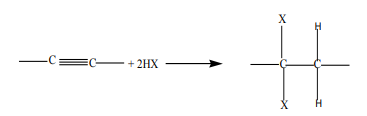
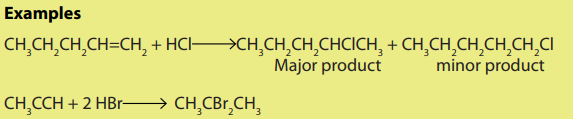
ii. Halogens

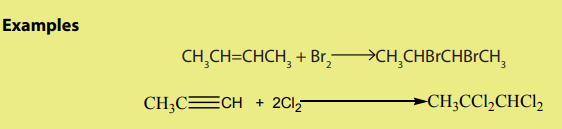
2. From alcohols
When ethanol reacts with potassium bromide in the presence of concentrated
sulphuric acid, bromoethane is formed. The reactions that took place in flask A arethe following.
In this reaction the hydroxyl group –OH is replaced with a bromine atom.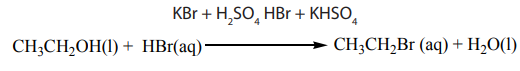
Halogenoalkanes are also obtained from alcohols using other reagents such asphosphorus halides.
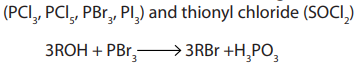

3. From alkanes
Activity 4.4.2
Observe the set up below and answer to the following questions.
Record your answers and discuss.
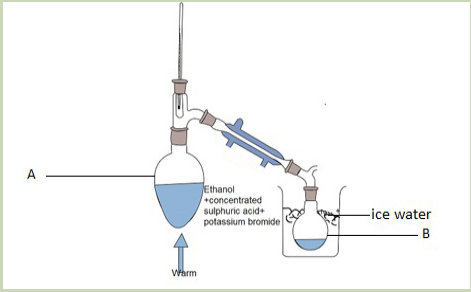
Picture 4.2: preparation of halogenoalkanes
a. Observe carefully the picture and suggest the product of the reaction in
flask A.
b. What is the role of:
i. Concentrated sulphuric acid?ii. Water in the picture above?
Direct halogenation of alkanes in the presence of ultraviolet light
gives alkyl halides and a hydrogen halide.
Example: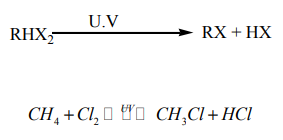
4. From aldehydes or ketones
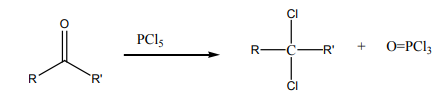

Checking up 4.4:
1. Complete the following chemical reactions :
2. Give the reagents and conditions needed to make the following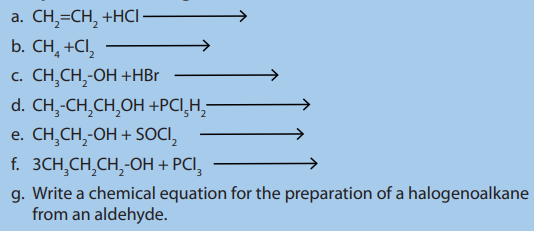
compounds from 1-bromopropane:a) propan-1-ol, b) propene.
4.5. Chemical properties
Activity 4.5.1
To investigate some reactions of halogenoalkanes
To of dilute sodium hydroxide solution in a test tube,
of dilute sodium hydroxide solution in a test tube,
add 5 drops of 1-bromobutane and gently warm the mixture.
Carefully smell the product.
Neutralize the solution with dilute nitric acid. Acidify the solution by adding 5
more drops of nitric acid.
Then add 5 drops of silver nitrate and observe. Write down your observations.
Write the equation of the reactions that take place. What is the role of sodium
hydroxide in this experiment?
When 1-bromobutane reacts with dilute sodium hydroxide solution, a product
with a sweet alcoholic smell is formed. That indicates that an alcohol is formed.
The formation of a pale yellow precipitate on addition of silver nitrate indicates
the presence of bromide ions. That means the carbon-bromine bond has been
heterolytically broken (the bromine atom takes the whole bonding electron pair). In
other words the bromine atom has been replaced by hydroxide ions. Thus, sodium
hydroxide provides the OH- ion which replaces bromine atom which leaves as a
bromide ion. As OH is a nucleophile (Lewis base) this reaction is called nucleophilicsubstitution.
The order of reactivity for the same alkyl group is such that iodides > bromides >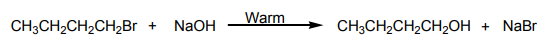
chlorides >fluorides.”>”, “are more reactive than “
The greater the electronegativity of the halogen, the greater the separation of charges
on the carbon and the halogen atoms, hence the stronger the bond. Therefore the
reaction is fastest with Iodoalkane because iodine is less electronegative compared
to bromine and Cl. Hence it will have weak C-I bond unlike that of C-Cl which will be
very strong due to the strong electronegativity of the chlorine atom. Hence bondenergies below are due to the above reason.
Because the carbon atom attached to the halogen atom is deprived of its electron, it
carries a partial positive charge . Thus when electron rich substrates
. Thus when electron rich substrates
called nucleophiles, approach the carbon atom, the halogen atom leaves as a halide
ion. Hence alkyl halides undergo nucleophilic substitution reaction, also writtenas SN.
1. Nucleophilic substitution reaction:

a. Reaction with aqueous alkali:
when alkyl halides are refluxed with aqueous alkali, or moist silver oxide, alcoholsare produced through substitution of the halogen by hydroxide ion.
This reaction is also called “hydrolysis”

Example:
Note: Tertiary alkyl halides react by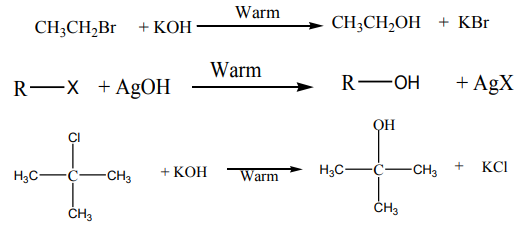
 mechanism, i.e. the mechanism where
mechanism, i.e. the mechanism where
the first step is the self ionization forming a carbonium ion (carbocation), an alkyl
radical that has lost its electron and bear a positive charge on the carbon, which
immediately adds the nucleophile
Secondary alkyl halides however react by either mechanism depending
mechanism depending on the condition of the reaction while primary alkyl halides react by
 (see below).
(see below). : Unimolecular Nucleophilic Substitution that takes place in two steps; the
: Unimolecular Nucleophilic Substitution that takes place in two steps; the reaction rate is determined by the concentration of one molecule.
Example of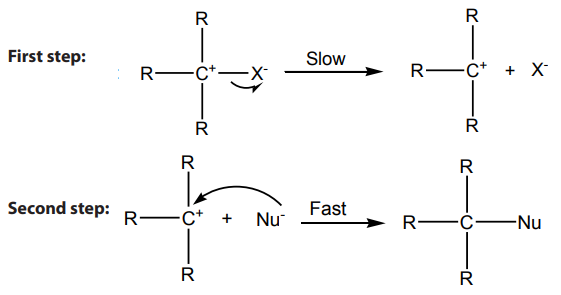
 reaction: hydrolysis of tertiary alkyl halides with sodium
reaction: hydrolysis of tertiary alkyl halides with sodium hydroxide.
The hydrolysis can also take place when water alone is added to tertiary alkyl halides.
In this case water molecules act as nucleophiles.

Mechanism:
Step 1: Self ionization of the alkyl halides to a stable carbonium ion and a halide ion.This is the slowest step of the reaction hence it is the rate determining step.
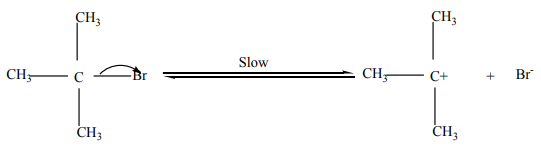
Step 2: Attack by incoming nucleophile. This is a fast reaction.
The potential energy (P.E )against reaction co-ordinate for hydrolysis of tertiary alkyl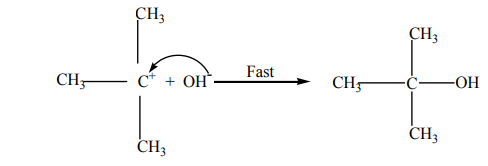
halides is as below (Fig.4.2). Potential energy is the energy stored in chemical bonds
of a substance, or the energy of an object due to its position.
The diagram below shows that the products formed have lower energy than thereactants, this indicates a favorable situation for the reaction to occur spontaneously.
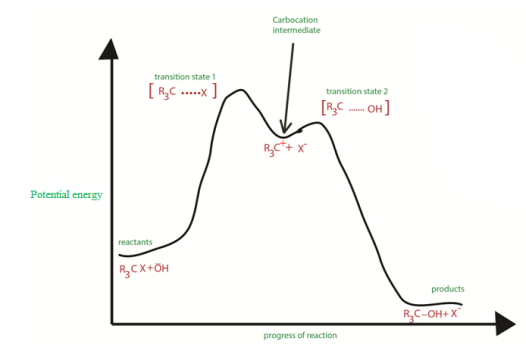
Figure 4.2:
The potential energy of the system initially increases because the energy is required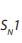 potential energy diagram
potential energy diagram
to break C-X bond; but when the stable carbocation is formed, energy is released
and the potential energy decreases a bit. As the carbocation and the nucleophile
(OH-) require a minimum energy (activation energy) to collide efficiently, the P.E
rises again until the transition state is reached, where the carbon-oxygen is being
formed. When this bond is completely formed, energy is released and the potentialenergy decreases.
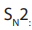 : Bimolecular Nucleophilic Substitution that takes place in one step; the
: Bimolecular Nucleophilic Substitution that takes place in one step; the
reaction rate depends on the concentration of Nu-
and the concentration of R-X.
In this mechanism, contrary to mechanism, the intermediate state also called
mechanism, the intermediate state also called
“activated complex” comprises both the leaving group and the entering group: inthe reaction below, the leaving group is X whereas the entering group is Nu.

b. Reaction with sodium alkoxides
Treatment of alkyl halides with sodium alkoxides produces ethers(Wiliamson synthesis)
c. Reactions with silver salt of carboxylic acid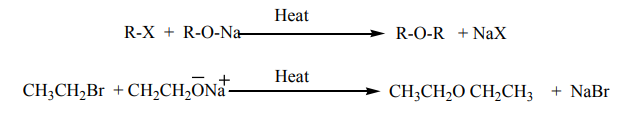
When alkyl halides are refluxed with silver salt of carboxylic acid, esters are formed:
(d) Reaction with potassium cyanide
When alkylhalides are refluxed with KCN, in presence of an alcohol, alkyl nitriles areproduced.
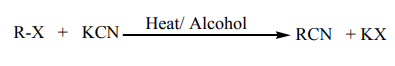
Example:
Note: This reaction is of practical importance in organic synthesis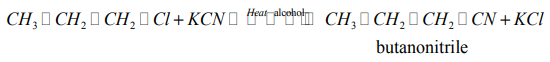
because it is used to increase the length of a carbon chain.e. Reaction with silver nitrite
When alkyl halides are refluxed with silver nitrite, a mixture of a nitro alkane and
alkyl nitrite are obtained as the products. The difference between the two products
is in the bonds between the nitrite and the alkyl: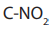
in nitro alkane and C-ONO in alkyl nitrite.
The two products can be separated by fractional distillation.
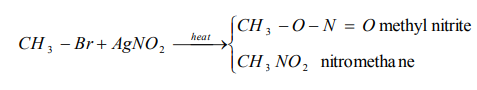
f. Reaction with ammonia and amines
Reaction of alkyl halide with concentrated ammonia produces a mixture of amines

The alkyl amine produced can then react with a molecule of alkyl iodide to producea series of substituted amines as shown in the reactions below:

Example of
 reaction: hydrolysis of primary alkyl halides with sodium hydroxide
reaction: hydrolysis of primary alkyl halides with sodium hydroxide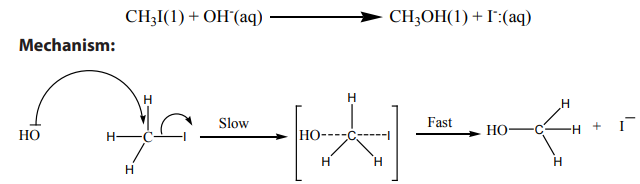
The transition state shows partial C-O bond formation and partial C-I bond cleavage.
Energy change diagram during the reaction is as below:
The P.E of the system initially increases along AB curve because energy is required
to break C-I bond; but when C-O is formed, energy is released and this is shown by
the curve BC. Since energy released by the formation of C-O bond is greater than the
energy required to break C-I, the products end up with a lower energy compared
to the reactant and this is favourable for the reaction to occur. At B a maximum
P.E is reached when C-I bond is partially broken and C-O bond is partially formed.
This state is called the transition state or activated complex. The energy barrier,
Ea, which must be overcome in order that the transition state is reached, is called
the activation energy of the reaction. The P.E of the system then falls along BCreleasing energy due to the formation of C-O bond.
Primary alkyl halides prefer reaction because of the unstable nature of the
reaction because of the unstable nature of the
intermediate or the activated complex formed in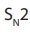 mechanism, the primary
mechanism, the primary carbonium ion,
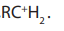
Table: 4. 1: Summary of alkyl halides reactions.

2) Elimination reactions
Activity 4.5.2
1. What is meant by elimination reaction?
2. Can halogenoalkanes undergo elimination reactions? Explain.
3. a. What are the products of an elimination reaction in halogenoalkanes?
b. What specific name is given to this reaction?
c. What are the conditions and reagent required for this type ofreaction?
An elimination reaction is where a saturated organic compound loses an atom
or group of atoms to form an unsaturated organic compound. Elimination is the
opposite of addition reaction.
Alkyl halides when boiled with alcoholic potassium hydroxide form alkenes byelimination reaction. Hence the alkyl halide loses a molecule of the hydrogen halide.
Note: Elimination reaction usually occurs in competition with substitution reaction.
So when chloroethane is treated with a solution of potassium hydroxide two organicproducts are formed depending on the conditions of the reaction.
Ethene is formed by elimination reaction while diethyl ether is formed by substitution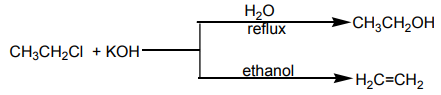
reaction.
Ether is formed by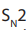 mechanism in which
mechanism in which 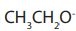 is acting as nucleophile.
is acting as nucleophile.
while Ethene is formed by elimination reaction in which is acting as base?
is acting as base?
Mechanism:

Because the two molecules are involved i.e.
and the reaction is bimolecular and since the alkyl halide loses a mole of HCl
the reaction is called elimination. Hence the reaction is a bimolemolecular elimination (E2).
In competition between and E2 in primary or secondary alkyl halides, the nature
and E2 in primary or secondary alkyl halides, the nature
of the product formed depends on the solvent, temperature, and structure of thehalide.
Elimination is favoured by use of high temperature and a strong base e.g alcoholinstead of water.
For tertiary alkyl halides, elimination occurs by E1 mechanism. In the mechanism,the tertiary alkyl halide undergoes ionization first and then later loses a proton.
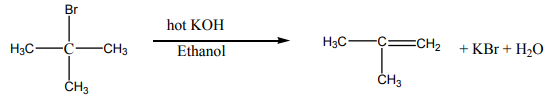
3) Wurtz reaction
Alkyl halides with sodium metal to give alkanes.
These are compounds in which more than one halogen atom is present. There are
two main types of dihalides.
Gem dihalides: This is where two halogen atoms are attached to the same carbonatom.
Example
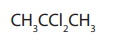
Vicinal dihalides: Here the two halogen atoms are on adjacent carbon atoms
Example
The reactions of dihalogenoalkanes are similar to those of monohalogenoalkanes
but require more reagents.
Examples
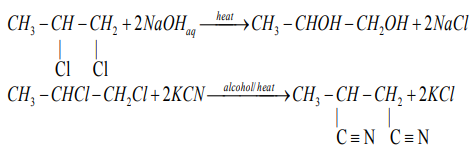
Elimination reaction with excess hot alkali produces alkynes

Checking up 4.5
1. Give the structural formula of the main product of each of the followingreactions:
2. Halogenoalkanes undergo nucleophilic substitution reaction. Discuss

this statement.
3. a. What is a nucleophile? Give two examples.
b. Why do nucleophiles attack halogenoalkanes?
c. What two types of reaction are in competition when a
halogenoalkane reacts with a nucleophile? Name two products
which can be formed from 1-bromopropane by these reactions.
4. 2- Chloro-2-methyl propane reacts with aqueous sodium hydroxide to
form 2-methylpropan-2-ol.
a. Draw what should be the energy diagram for the reaction.
b. Write the mechanism for the reaction.
c. (i) Sketch an energy diagram for the reaction of aqueous sodium
hydroxide and chloromethane.
(ii) Outline the mechanism for the reaction.
d. outline the mechanism for the reaction
4.6. Chemical test for the presence of halogenoalkanesActivity 4.6:
Put 2mL of ethanol into each of 4 test tubes labelled A-D. A is the control tube and
therefore no alkyl halides are to be added. To B, add 3-4 drops of 1-chlorobutane.
To C, add 3-4 drops of 1-bromobutane and to D, add 3-4 drops of 1-iodobutane
using Pasteur pippete. Stopper the tubes and place them in a hot water bath at
about
and leave for a few minutes to equilibrate. Working quickly add about
1mL of silver nitrate solution to each tube. Start the stopwatch and shake the
tubes to ensure complete mixing.
a. Record your observations
b. Make a comment about comparison of the reactions of the threehalogeno alkane
Halogenoalkanes can be identified due to some tests. The following Table illustratesome chemical tests of halogenoalkanes.
Table 4. 2: Chemical test for halogenoalkanes

Checking up 4.6
Given two samples A and B. You carry out the test for haloalkanes and get the
following results: A form a pale yellow precipitate and B form a white precipitate.
Which sample represents and which one represents
and which one represents 
Write chemical equations to justify your answer.
4.7. Uses of halogenoalkanes and dangers associated with CFCs
Activity 4.7
1. Do you know CFCs? If yes what do you know about them?
2. Do CFCs affect directly our health in our daily life? If yes explain how.
3. What are the dangers posed by CFCs?
4. What solutions do you propose or have been proposed to the problemof CFCs
Solvents:

Medicine:
 is used in anesthesia
is used in anesthesiaAgriculture:
• DDT: Dichloro diphenyl trichloroethane is used as insecticide- DDT. Colorless
chemical pesticide, dichlorodiphenyltrichloroethane, used to eradicate
disease-carrying and crop-eating insects. It was first isolated in Germany
in 1874, but not until 1939 did the Swiss Nobel Prize-winning chemist Paul
Müller recognize it as a potent nerve poison on insects. The product is bannedin Rwanda. Below is the structure of DDT.
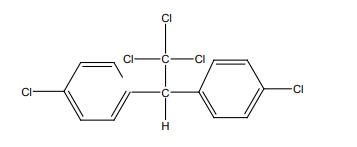
Home: Refrigeration, perfumes, etc…
Halogenoalkanes which have boiling temperatures just below room temperature
can easily be liquefied by a slight increase in pressure. Halogenoalkanes containingchlorine and fluorine and no hydrogen are Chlorofluorohydrocarbons. Examples are
 They are usually called chlorofluorocarbons or CFCs. In
They are usually called chlorofluorocarbons or CFCs. In
addition to having low boiling temperatures, they are non-flammable, odorless,stable, non-toxic and solvents.
• CFCs appeared to be ideal for use as fluids in refrigerators and as solvents
in aerosol sprays, they were developed in the 1920s as what appeared to
be ideal replacements for liquid ammonia and liquid Sulphur dioxide, which
were formerly used as fluids in refrigerators and air-conditioning units. Being
good solvents, they were also ideal as the solvents in aerosol sprays.
Aerosols were used to dispense insecticides, hairsprays, perfumes and deodorants,
window-cleaning, polishes, waxes and laundry products. As more and more
uses were found for these remarkable compounds, CFCs became big business,
with hundreds of thousands of tones being produced yearly. Now theyare being phased out. These stable, non-toxic compounds are dangerous!
•Their high stability has turned out to be a problem, during all the time that
the use of CFCs was increasing, no-one thought about what would happen to
the gases in the atmosphere. Because of their lack of reactivity and insolubility
in water, there is no natural process for removing CFCs. In fact they drift up
into the stratosphere where ultraviolet light causes photolysis, i.e. a reaction
cause by light. The chlorine radicals formed in photolysis take part in a chainreaction which converts ozone into oxygen.
As you can notice, the chain of reaction above results in the decomposition of ozone
,which does have the capacity to absorb, and stop dangerous UV from reaching the
Earth into ordinary oxygen. This can be avoided if and only if human activities sendno CFCs in the atmosphere.
• This reduce the thickness of the ozone layer. Reactions (a) to (c) form a chain.
This is why one chlorine radical from one CFC molecule can destroy thousandsof ozone molecules.
And what can be done?
• Replacements for CFCs have been found, because of concern over the
decrease in the ozone layer, many nations have agreed to cut down the use
of CFCs. Alternative compounds are already in production. Hydrohalocarbons
contain at least one hydrogen atom per molecule. The C-H bond can be
attacked by HO• radicals in the lower atmosphere and the compounds do notreach the upper atmosphere. Hydro halocarbons include
• Hydrochlorofluorocarbons, used in blowing plastics
used in blowing plastics
foam and used in air-conditioners
used in air-conditioners
• Hydrofluorocarbons, used in air-conditioners and
refrigerators.
used in air-conditioners and
refrigerators. HCFs cause no damage to the ozone layer, although they are greenhouse gases.
Checking Up 4.7
1. State four industrial uses of the halogenoalkanes. Why do fluoroalkanesfind special uses?
4.8. End Unit Assessment
1. Which of the following is NOT a halogenoalkane compound:
a. Tribromo benzene
b. 3-iodohexane
c. 2-chloro-3-methylpentaned. 2-bromopentane
2. Choose from a list of words and fill in the missing words in the text
below
Halogenoalkane, iodine, alkyl halide, haloarene, thyroxine
…………………………..compounds are compounds in which the
halogen atoms like chlorine, bromine, ………… or fluorine are
attached to a hydrocarbon chain or an aromatic ring. When the halogen
atom is attached to a hydrocarbon chain the compound is called an…………………… or ………………………..
3. Answer by True or False
a. Chloroform is employed as a solvent in paint remover.
b. Iodoform was used earlier as an antiseptic.
c. Methyl chloride, methyl bromide, ethyl chloride and some
chlorofluoromethanes are gases at room temperature.
d. The objects which are non-superimposable on their mirror image
(like a pair of hands) are said to be chiral and this property is known
as chirality. While the objects, which are superimposable on theirmirror images are called achiral.
e. (chloroform): is used as insecticide
(chloroform): is used as insecticide
f. DDT: Dichloride diphenyl trichloroethane is used as anesthesia
g. Halogenoalkanes therefore, although they dissolve more than
alkanes, are only slightly soluble in water.
h. Halogenoalkanes undergo nucleophilic substitution reactions in
which the halogen atom is replaced by a nucleophile.
i. Elimination reaction is where a saturated organic compound loses
an atom or group of atoms attached to form unsaturated organiccompound.
4. Name the following halides according to IUPAC system and classifythem as primary, secondary or tertiary halogenoalkanes
1. Write the structures of the following organic halogen compounds.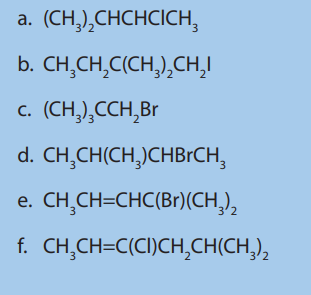
a. 2-chloro-3-methylpentane
b. 2-chloro-2-methylpropane
c. 2,3-dichlorobutane
d. 2-bromo-4-chloropentanee. 1,1,2-trichloropropane
2. Why do bromoalkanes react more readily than chloroalkanes?
3. Why does 1-bromopropane react with nucleophiles but propane doesnot?
4. Write the equations for the preparation of 1-iodobutane from
(a) 1-butanol, (b)1-chlorobutane, (c) but-1-ene
5. Write the structure of the major organic productin each of the following reactions:
6. Arrange the compound of each set in order of reactivity towards SN2
displacement:
a. 2-bromo-2-methylbutane, 1-bromopentane, 2-bromopentane
b. 1-bromo-3-methylbutane, 2-bromo-2-methylbutane, 3-bromo-2-
methyl butane
c. 1-bromobutane, 1-bromo-2,2-dimethylpropane, 1-bromo-2-methyl butane, 1-bromo-3-methylbutane.
7. a) There are four strucural isomers of molecular formula C4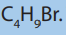 The formulae of two of these isomers are given.
The formulae of two of these isomers are given.
i. Draw the remaining two structural isomers.
ii. Give the name of isomer 2
b) All four structural isomers of
i. Give the name of the mechanism involved in these reactions.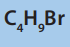 undergo similar reactions with
ammonia
undergo similar reactions with
ammonia
ii. Draw the structural formula of the product formed by the reaction
of isomer 2 with ammonia.
iii. Select the isomer of molecular formula C4
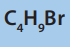 that would be most
that would be most
reactive with ammonia. State the structural feature of your chosen
isomer that makes it the most reactive of the four isomers.
iv. The elimination of HBr from Isomer 1 produces two structural
isomers, compounds A and B.
v. Give the reagents and conditions required for this elimination
reaction.
c) Ethene, C2H4, reacts with bromine to give 1,2-dibromoethane.
i. Give the name of the mechanism involved.
ii. Show the mechanism for this reaction.
vi. Give the structural formulae of the two isomers, A and B formed byelimination of HBr from isomer 1.
UNIT 5: ALCOHOLS AND ETHERS
Key unit competency:
To be able to compare the physical and chemical properties of alcohols and ethers totheir preparation methods, reactivity and uses.
Learning objectives:
• Distinguish between alcohols from other organic compounds by
representing the functional group of alcohols
• Classify primary, secondary and tertiary alcohols by carrying out the method
of identification
• Write the name of alcohols by using IUPAC system
• Describe the physical properties of alcohols to other series of organic
compounds
• Carry out the method of preparation of alcohols
• Describe the local process of making alcohol by fermentation.
• Explain the effect of oxidation on urwagwa when it overstays
• Compare the physical, chemical and the method of preparation of alcohols to
ethers• State the use of ethers
Introductory activity
The following represent two pictures or pictures A and B. Observe each picturecarefully and answer to the related questions.

1. Discuss the meaning of each picture.
2. What objects do you observe in the above pictures?
3. Explain the consequences that can arise from the picture B
5.1. Definition and nomenclature
Activity 5.1
1. Look at the following compounds and classify them in theirhomologous series.
2. By doing your own research, distinguish the rules used to name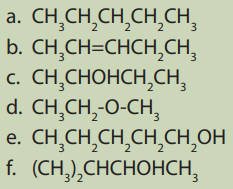
alcohol compounds.
5.1.1. Definition
Alcohols are organic compounds that are derivatives of hydrocarbons where one
or more hydrogen atoms of hydrocarbon is or are replaced by hydroxyl (-OH) group.They are represented by the general formula:
where R is a radical: alkyl group made by a chain of carbon atoms
Alcohols are called monohydric if only one hydroxyl group is present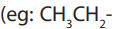
 Dihydric alcohols are those with two hydroxyl group
Dihydric alcohols are those with two hydroxyl group
(diol: vicinal and gem), trihydric (triols) and polyhydric are those with many – C-OH groups.
The functional group attached is –OH group to any atom of carbon.5.1.2. Nomenclature
According to IUPAC system, alcohols are named by replacing the final ‘‘e’’ of the
parent hydrocarbon with ‘‘ol’’, then specify the position of -OH group before endingby ol.

When there are more than one hydroxyl group present, prefixes, di, tri, tetra,... are used.

 1. Write the structural formulas of all organic compounds containing
1. Write the structural formulas of all organic compounds containingNotice: -OH group takes priority over alkyls substituents, double or triple bonds and
even halides.

Checking up 5.1 According to IUPAC system, name each of the following compounds:
5.2. Classification and isomerism
Activity 5.2
C-OH group and fit the molecular formula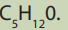
2. Based on their structures and your knowledge about classes of
halogenoalkanes, classify the compounds identified in 1) above.
3. Classify them as chain and position isomers.4. Which of them can exhibit optical isomerism?
Alcohols are classified as:
Primary alcohols: These have only one alkyl group attached to the carboncarrying the –OH.
Examples
Secondary alcohols: they are alcohols in which the OH group is attached to carbon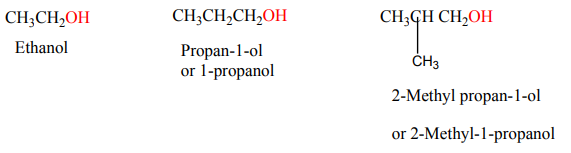
atom bonded to two other carbon atoms.
Tertiary alcohols: they are alcohols in which the OH group is attached to carbon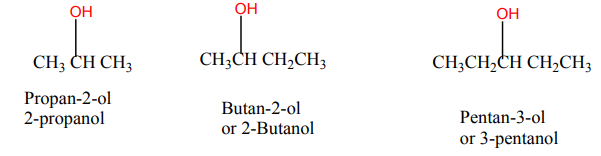
atom bonded to three other carbon atoms.
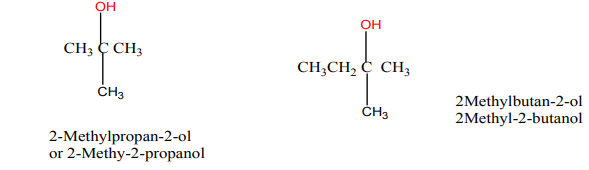
Alcohols containing at least three carbon atoms exhibit different types of isomerism:
• Chain isomerism:
This is due to the difference in the size of the chain.

• Position isomerism:
This is due to different positions taken by the –OH in the same carbon chain.

• Functional isomers:
Except methanol which has one carbon, other alcohols are isomers with ethers
another chemical function of general formula R-O-R’
where R and R’ are alkyl groupsor aryl groups but not hydrogen.
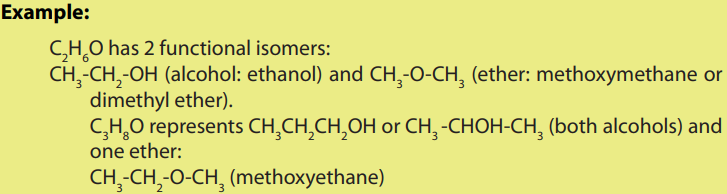
5.3. Physical properties
Activity 5.3.
Analyze the following data and answer to the question
Explain the trends in the boiling point of the molecules given in the table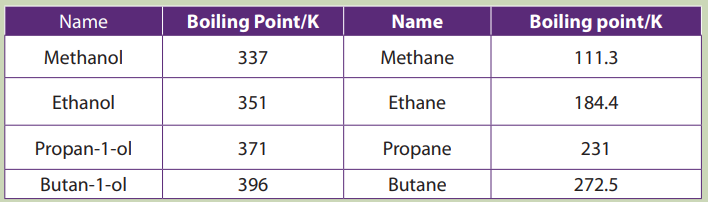
Compare and explain the differences in the boiling point of alkanes and alcohols
a. Boiling points
The chart shows the boiling points of some simple primary alcohols and alkaneswith up to 4 carbon atoms.
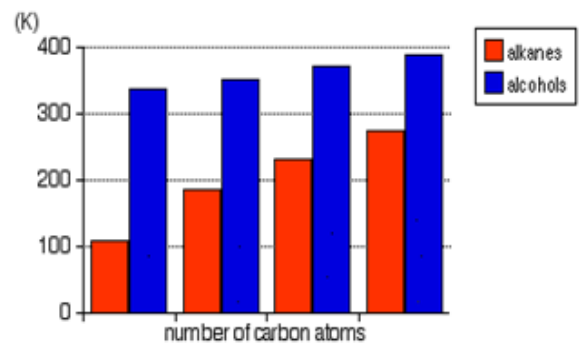
Figure 5.1: boiling points of alcohols and alkanes
• The boiling point of an alcohol is always much higher than that of the alkane
with the same number of carbon atoms.
• The boiling points of the alcohols increase as the number of carbon atoms
increases.
• The boiling point of alcohols with branches is lower than that of unbranched
alcohols with the same number of carbon atoms. This is because increased
branching gives molecules a nearly spherical shape and the surface area
of contact between molecules in the liquid. This results in weakened
intermolecular forces and therefore in lower boiling points.
• Tertiary alcohols exhibit the lowest boiling point than secondary and primary
alcohols:
• Primary alcohol > Secondary alcohol > Tertiary alcohol
Highest boiling point lowest boiling point
The patterns in boiling point reflect the patterns in intermolecular attractions:
In the case of alcohols, there are hydrogen bonds set up between the slightly positivehydrogen atoms and lone pairs on oxygen in other molecules.
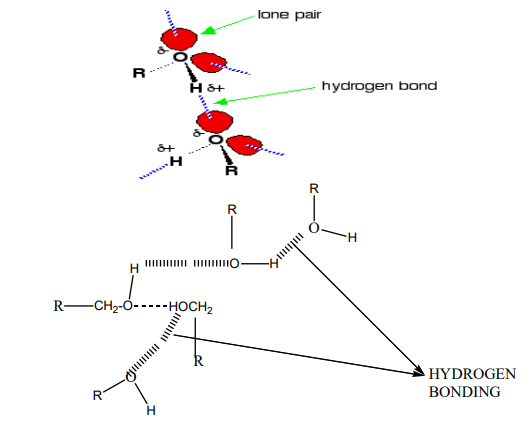
b. Solubility of alcohols in water
The lower members of alcohols are completely soluble in water because mixed
hydrogen bonds between water and alcohol molecules are formed. As the length ofhydrocarbon group of the alcohol increases, the solubility decreases.
c. Volatility
Alcohols are volatile and the volatility decreases as the molecular mass increases.
Compared to alkyl halides, alcohols are less volatile. Polyalcohol are viscous or solids.
Example: propane-1, 2, 3-triol (glycerine). This is due to stronger intermolecularforces than those of mono alcohols.
Checking up 5.3
1. Comment on the solubility of alcohols compared to alkanes in water.
2. Ethanol with a molecular mass of 46 and butane with a molecular mass
of 58 have the boiling point of
 respectively.
respectively. Explain these differences.
3. Are alcohols electric conductors? Justify your answer.
5.4. Alcohol preparations
Activity 5.4
Complete the following chemical equations. For each, show the mechanism ofthe reaction
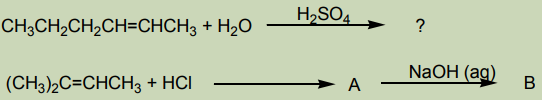
Alcohols are prepared with different methods
a. From alkyl halides
Alkyl halides when refluxed with aqueous alkali (NaOH or KOH) or moist silver
oxide (Ag OH) produce alcohols. The hydrolysis occurs by a nucleophile substitutionreaction.
Note: During the reaction of these preparations of alcohols, you have to use the
dilute NaOH, KOH and warm in order to increase the rate of
for primary alcohol while tertiary alcohols undergo
b. From alkenes
Alkenes react with water in the presence concentrated sulphuric acid to yieldsalcohols
Notice: Alkenes in the presence of Aluminum oxide reacts with water to form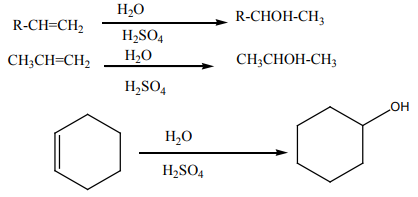
alcohols in vapour phase then condense to give liquid alcohols.
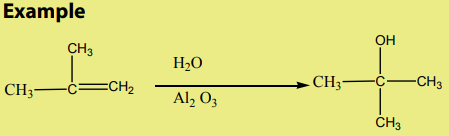
c. From carbonyl compounds
When aldehydes and ketones are reduced by hydrogen in the presence of a suitablecatalyst like Pt, Ni or Pd, they form primary and secondary alcohols respectively.
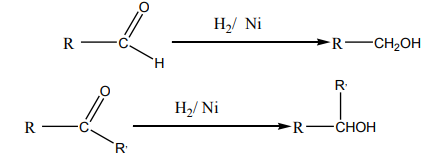
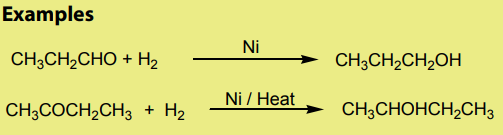
Note: Lithium tetrahydridoaluminate (LiAlH4 ) can also be used as a reducing agent.
Lithium tetrahydridoaluminate is not a stronger enough as reducing agent to reduce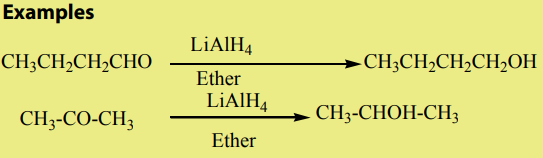
a double bond unlike
which can reduce both the double bond and the carbonyl group.
Activity 5.5
Process of alcoholic fermentation
d. From esters
Esters on hydrolysis in the presence of mineral acid or alkalis produce alcohols andcarboxylic acids.

Note:
group followed by hydrolysis.e. From Grignard reagents
The reaction between carbonyl compound and Grignard reagent (alkyl magnesium
halides) produces an alcohol with more carbon atoms. The reaction is a nucleophilicaddition on a carbonyl compound.
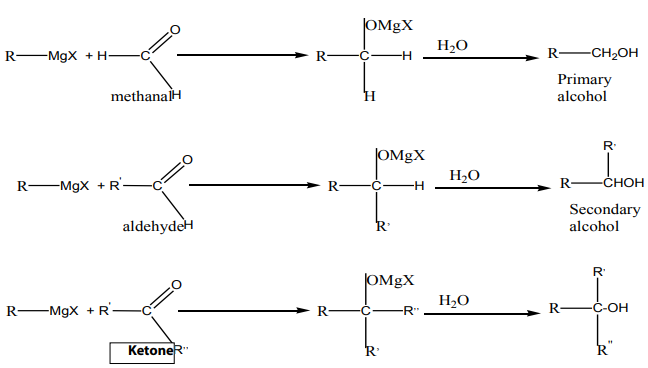

f. From primary amine to give primary alcohol
Primary amines react with nitrous acid to produce primary alcohols.
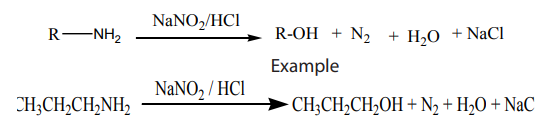
Checking up 5.4
1. Using chemical equations, explain how 3-methylbutan-2-ol could be
prepared:
a. from an alkene
b. using a Grignard reagent
c. from a halogenoalkanes
d. from an aminee. by reduction of a carbonyl compound
5.5. Preparation of ethanol by fermentation
Activity 5.5Process of alcoholic fermentation
6. Observe the above pictures and then interpret each picture.

7. Describe the process followed to produce alcohol refer to the above
pictures.
a. What are the raw materials used in the process?
b. What is the main component of the final products?
c. Give the name of the process illustrated by the pictured. Propose another process that can be used to yield the product in b.
This method is mainly used to prepare ethanol industrially. Ethanol is prepared from
starch (e.g. maize, cassava, millet, sorghum) and sugar (e.g. banana juice, molasses)
by fermentation process.
Fermentation can be defined as any of many anaerobic biochemical reactions
in which enzymes produced by microorganisms catalyse the conversion of onesubstance into another.
Alcoholic fermentation is the process in which enzymes act on carbohydrates to
give simpler compounds like ethanol (alcohol) and carbon dioxide
a. From starch
malt obtained either from maize grain, millet, or cassava contains an enzyme calleddiastase which catalyzes the hydrolysis of starch to maltose.
At room temperature, yeast is added and one of its enzymes called maltase catalyzes
the hydrolysis of maltose to simple sugar so called glucose.
Finally another enzyme of yeast called zymase catalyzes the decomposition of
glucose to ethanol.
b. From sugar
Molasses containing sugars are mixed with water and yeast and then allowed to
ferment for several days after which ethanol are obtained during fermentation
process.
One enzyme of the yeast called sucrase catalyzes the hydrolysis of sucrose presentin the molasses to glucose and fructose.
Thus, another enzyme of yeast called zymase catalyzes
the decomposition of glucose to ethanol.
The ethanol obtained by fermentation process is only about 11%. This is made
concentrated by distillation which converts it to about 95% ethanol. This on further
distillation yields a constant boiling mixture whose composition does not change
(an azeotropic mixture).
Therefore, 100% ethanol is obtained by either:
i. Adding quick lime which removes water
ii. Distilling with of benzene as a third component
Note: Methanol can be prepared industrially by the reaction of carbon monoxide
and hydrogen at 300 °C and a pressure of 200 atmospheres.

Checking up 5.5
1. Briefly, describe the preparation of ethanol by alcoholic fermentation.
2. Compare and contrast the preparation of ethanol by hydration of etheneand by alcoholic fermentation.
Project work 5.1
The task is about the fermentation of glucose
Part A
In this project, you will investigate the fermentation of different types of
substances containing starch.
Requirements
• Conical flask,
• some yeast,
• some boiled potatoes,
• some bread,
• some boiled cassava,
• boiling tube,
• cork with delivery tubes,
• stands and cramps,
• glucose,• weighing balance
Why boiled potatoes or cassava is preferred?
Procedure
Weigh 25g of each starch including glucose and place them in separate conical
flasks. Add to each one spatula of dried yeast followed by 100 cm3
of water.
Cork the conical flask and connect it to boiling tube containing lime water as
shown in the figure below. Label each flask clearly. Leave all the flasks in a warm
environment. Record your observation for seven days.
c. The conical flask
d. The boiling tubeFor each type of starch
Note: use the same quantity and concentration of lime water in each boiling
tube.
Part B: Comparison of yield of alcohol obtained
Filter the mixture from each flask separately and collect the filtrate in measuring
cylinder. Record the volume in each case. Perform fractional distillation on each
filtrate, collecting the fraction between Record the volume of
Record the volume of
distillate collected from each starch.
Take of each on watch glass, ignite and note the time it takes to burn completely.
of each on watch glass, ignite and note the time it takes to burn completely.
Observe the amount of water left on watch glass.
5.6. Chemical properties of alcohols
Activity 5.6.1.
To investigate the oxidation reaction of an alcohol.
Requirements: methanol, ethanol, 2M sulphuric acid, potassium dichromate
solution, test tubes, burner, droppers, propan-2-ol and 2-methylpropan-2-ol.
Procedure:
• Place 5 drops of methanol in test tube
• Add 10 drops of dilute sulphuric acid followed by 5 drops of potassium
dichromate solution.
• Warm the mixture gently
• Repeat the experiment with ethanol, propan-2-ol and 2-methylpropan-2-ol.
1. What happens to the colour of the solution?
2. Explain the observation.3. Write an equation for the reaction taking place.
5.6.1. Oxidation
Primary and secondary alcohols are oxidized to aldehydes and ketones respectivelyby use of acidified.
 nitric acid once concentrated.
nitric acid once concentrated.
Aldehydes formed by oxidation of primary alcohols tend to undergo further
oxidation to carboxylic acid.
Ketones formed by oxidation of secondary alcohols are not further oxidized, unless
if the oxidizing agent is hot and concentrated in which case bonds around the –
CO_ group are broken and two smaller carboxylic acids are formed.

Tertiary alcohols resist oxidation because they have no hydrogen atom attached on
the functional carbon atom. Oxidation also occurs when the alcohol is in gaseous
phase by used of silver or copper catalyst under 500 °C and 300 °C respectively;
and the vapour of the alcohol is passed with air (oxygen) over heated silver.Example
These reactions help to distinguish between primary, secondary and tertiary
alcohols because primary and secondary alcohols decolourise the purplesolution of
An acidified potassium dichromate solution is turned from orange to green when it
reacts with primary and secondary alcohols.
Secondary alcohols having the following structure only undergo
only undergo
oxidation, on treatment with iodine solution in the presence of sodium hydroxide togive yellow precipitate of tri-iodomethane.
Note: This is a reaction which is characteristic of methyl ketones,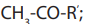 but
but
iodine here acting as an oxidizing agent first oxidizes the
then the methyl ketone formed then gives the yellow precipitate of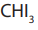 (Iodoform).
(Iodoform).From the reaction involved we have the Iodoform test.
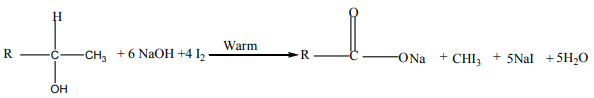
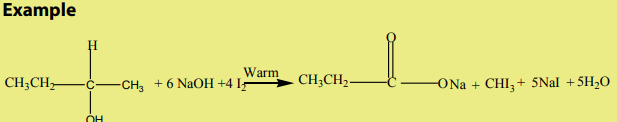
5.6.2. Reaction with sulphuric acid
Activity 5.6.2
Show the product of the reaction referring to the preparation of alkenes andshow the mechanism of reaction.
Alcohols react with concentrated acid to give products depending on the nature of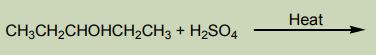
the alcohol and conditions of reactions.
a. At about 0 °C alcohols react with sulphuric acid to produce alkyl hydrogensulphates.
This reaction is a substitution reaction where the OH group has been replaced by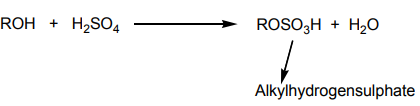

b. At about 140°C in the presence of excess primary alcohol and concentratedsulphuric acid, ether is formed.
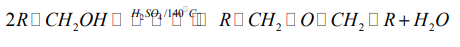
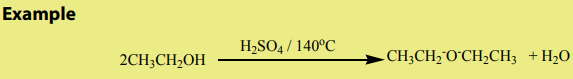
Mechanism
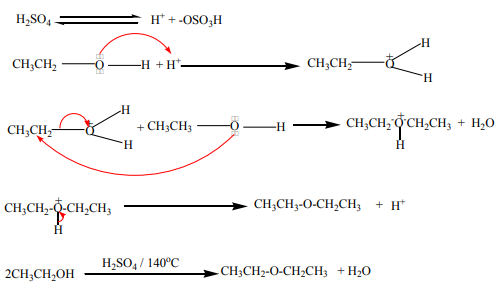
This reaction is an intermolecular dehydration.
c. Elimination reaction
Alcohols are dehydrated by heating with concentrated sulphuric acid or phosphoric
acid to alkenes. The ease of dehydration is in the order tertiary>secondary>primary’,this reaction is the intramolecular dehydration of water.
Notice: For primary alcohols any temperatures between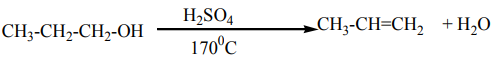
is sufficient and the acid should be sufficiently concentrated.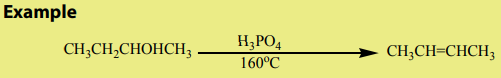
This dehydration respects Zaïtsev’s elimination law (see alkenes) reason why thehydration of butan-1-ol and butan-2-ol gives the same products which is but-2-ene via
Elimination always competes with nucleophilic substitution reaction. Substitution
leading to formation of ether is favoured by use of excess primary alcohols while
higher temperatures favour elimination. Therefore, dehydration of ethanol may
produce both alkenes by elimination and diethylether by substitution reaction. Therelative proportion of two products depends on the condition of the reaction.
Dehydration of alcohols also occurs when the vapours of the alcohols are passed
over heat aluminium oxide at about 300 °C.

5.6.3. Esterification
Alcohols react with organic acids in the presence of mineral acids lsuch as sulphuric
acid (catalyst) with elimination of water under 100 °C to produce an ester with givenoff a perfume smell.
This reaction is called “esterification”.
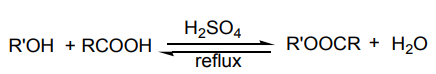
The mechanism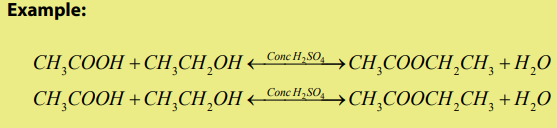
Step 1
In the first step, the ethanoic acid takes a proton (a hydrogen ion) from the
concentrated sulphuric acid. The proton becomes attached to one of the lone pairson the oxygen which is double-bonded to the carbon.
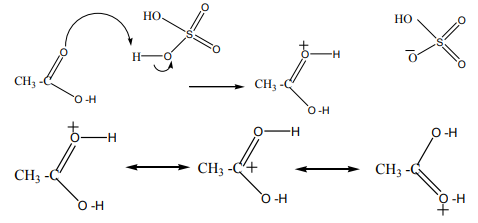
Step 2
The positive charge on the carbon atom is attacked by one of the lone pairs on theoxygen of the ethanol molecule.
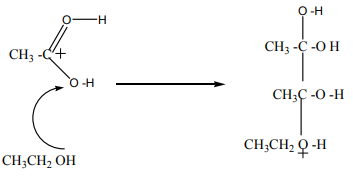
Step 3
What happens next is that a proton (a hydrogen ion) gets transferred from the
bottom oxygen atom to one of the others. It gets picked off by one of the other
substances in the mixture (for example, by attaching to a lone pair on an unreacted
ethanol molecule), and then dumped back onto one of the oxygens more or less atrandom.
The net effect is:
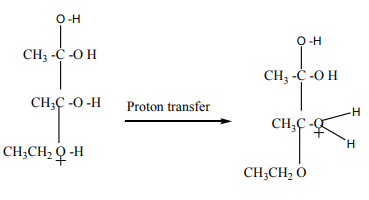
Step 4
Now a molecule of water is lost from the ion.
The product ion has been drawn in a shape to reflect the product which we are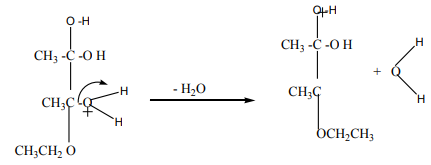
finally getting quite close to!
The structure for the latest ion is just like the one we discussed at length back in step
1. The positive charge is actually delocalised all over that end of the ion, and there
will also be contributions from structures where the charge is on the either of theoxygen atoms:
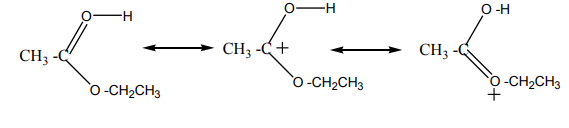
Step 5
The hydrogen is removed from the oxygen by reaction with the hydrogen
sulphate ion which was formed way back in the first step.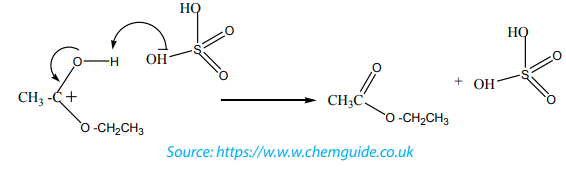
5.6.4. Reaction with strong electropositive metals and metal hydroxides
Electropositive metals like Na, K, react with alcohols forming alkoxide withevolution of hydrogen gas.

Note: Alcohols are not enough acidic to react with metal hydroxides such as
sodium hydroxide or potassium hydroxide.

5.6.5 Action of hydrohalic acids (HX)
Activity 5.6.5Referring to preparation of alkyl halides, complete the following reactions:
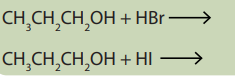
Alcohols react with hydrohalicacids to give alkyl halides.
Examples

Notice:
i. Reaction with concentrated hydrochloric acid is catalyzed by anhydrous
zinc chloride.
ii. This reaction is called LUCAS test and is used to distinguish between
simple primary, secondary or tertiary alcohols. In this reaction, the alcoholis shaken with a solution of zinc chloride in concentrated hydrochloric acid.
Observations: Immediate cloudiness indicates presence of a tertiary alcohol. If the
solution becomes cloudy within 5 minutes then the alcohol is a secondary one.
Primary alcohol would show no cloudiness at room temperature since the reactionis very slow.
For example all alcohols which are isomers of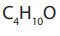 can be distinguished by the
can be distinguished by the LUCAS test.
Alcohols are also transformed into halogenoalkanes using phosphorus halides andthionyl chloride.
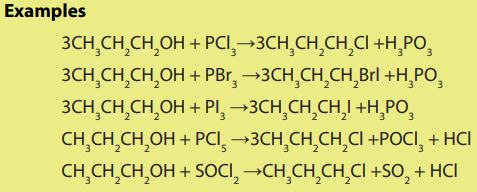
Checking up 5.6
1. An organic compound A possesses 87.6 % composition by mass and the
rest is hydrogen. If the same molecule possesses the molecular mass of
56 g/mol, deduce the molecular formula of A.
a) The reaction of A with water produces the compound B.
B can be represented in different forms called isomers. Represent theisomers of B.
When B reacts with it produces different compounds
it produces different compounds
depending on the reaction conditions. Write the structural formulae of
those compounds and state the conditions of their formation.
b) When B reacts with three products are obtained depending on
three products are obtained depending on the temperature used. Write structural formulae of those products.
2. Explain why tertiary alcohols are not oxidized.3. Complete the following chemical reactions and name the products obtained:

5.7. Uses of alcohols
Activity 5.7
In Rwnda, different types of alcoholic drinks are produced. However, some of
the them produced locally including “Kanyanga” are prohibited.
a. Explain why this alcohols is prohibited?
b. Discuss the possible effects of using non certified alcoholic drinks.
c. How would you differentiate alcoholic products from non-alcoholicones?
Ethanol is the alcohol found in alcoholic drinks. Alcoholic fermentation converts
starch sugar into ethanol. For example grapes are used to produce wine, ripe banana
to produce urwagwa, honey for spirits are obtained by distilling the ethanol –water
product obtained when sugar is fermented.
Drinking alcohol, i.e. the ethylic alcohol also called ethanol, is a normal social activity;
but excess of it is dangerous for our health. Hence excess of alcoholic consumption
must be avoided. For non-adult youth, consumption of alcohol in any form is illegalin Rwanda and many other countries.
There are some alcoholic drinks produced in Rwanda and in the Region that are
prohibited to be sold in Rwanda. However, alcohols have many other applications indaily life as indicated in the Table 5.1.
Table 5.1. Application of some alcohols

Ethanol produced by sugar cane fermentation has been used as alternative fuel to
gasoline (petrol). It has been mixed with gasoline to produce gasohol.
Project 5.2. USE OF ALCOHOLS
Consult leader to your community religious, political, professionals, e.g.
doctors, nurses, parents, teachers, and elders and find out from them thefollowing:
• What are the true recommended users of alcohols
• How should alcohol be used
• Real life examples of the effect of alcohol abuse on:
i. Social life
ii. Spiritual life
iii. Physical life of an individual
a. Come up with your own resolution and statements concerning alcohol
abuse. Write it out on a card and share it with trusted friends and your
parents/guardian and mentor.
b. Discuss with your peers show how you would help one of your members
of your family who is addicted to alcohol to come out of itc. Find out the good economic uses of alcohol.
5.8. Ethers
5.8.1 Structure and isomerism
Activity 5.8.1.
1. Represent the possible isomers of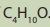 Which of them are:
Which of them are:
a. Structural isomers?
b. Functional isomers2. What homologous series do those isomers belong to?
Ethers are organic compounds in which two carbon groups are connected to a
single oxygen. The general formula of ether is R-O-R’. Based on the general structureof ether, they are classified as symmetrical, unsymmetrical and epoxide.
- For symmetrical esthers, R and R’ are identical
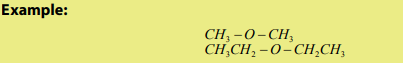
- For unsymmetrical esthers, R and R’ are different (R#R’)
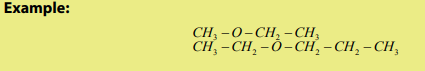
- Cyclic ethers
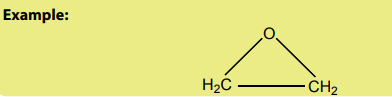
Checking up 5.8.
Classify the isomers of the molecules identified in activity 5.8.1 above intosymmetric, asymmetric and cyclic.
5.8.2. Physical properties
1. Ethers are sparingly soluble in water but are soluble in organic solvents.
2. The polar nature of the C-O bond (due to the electronegativity difference of
the atoms) results in intermolecular dipole-dipole interactions.
3. An ether cannot form hydrogen bonds with other ether molecules since
there is no H to be donated (no -OH group).
4. Their melting and boiling points increase with the increase in molecular
mass because of increasing the magnitude of Van der Waal’s forces with size.
5. The boiling points of ethers are much lower than those of alcohols of similar
molecular mass. This is because of the intermolecular hydrogen bondingwhich are present in alcohols but are not possible in ethers.
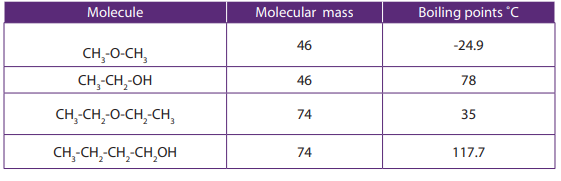
5.8.3 Preparation of ethers
Activity 5.8.2
1. With reference to alcohols, define intermolecular dehydration reaction.
2. State the reagent and conditions used in that reaction and give oneexample
1) Intermolecular dehydration of alcohols
This is done by heating excess primary alcohol with concentrated sulphuric acid orphosphoric acid at about 140˚C.
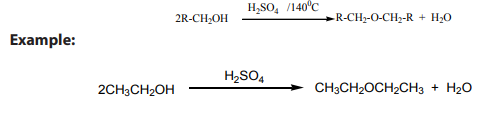
2) From halogenoalkanes
(a) In this method halogenoalkanes are heated together with sodium or potassiumalkoxides.
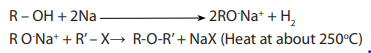
This is the Williamson’s synthesis
(b) In the second method, the halogenoalkane is heated with dry silver oxide.


5.9. Chemical properties of ethers
Activity 5.9
A compound with molecular formula has three isomers. One of them
has three isomers. One of themdoes not react with sodium metal. Identify that isomer.
Since they are saturated compounds and non-polar, they are relatively chemicallyinert reason why their chemical reactions are very few.
5.9.1. Reactions in which the carbon – oxygen bond is broken
a. Ethers react with hot concentrated sulphuric acid to form alcohols accordingto the following reaction.
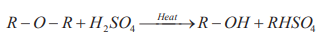
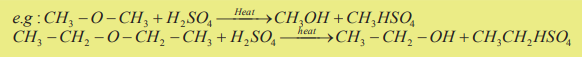
b. Reaction with hydrohalic acids
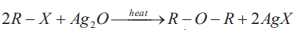
Ethers react with cold hydrohalic acids to form alkyl halides and alcohols.
R-O-R’ + HX→ ROH +R’X (cold)
Note: For unsymmetrical ethers, the halogen is attached to the smaller of the two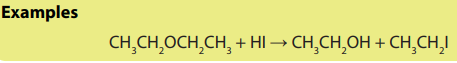
alkyl groups.

Ethers react with hot hydrohalic acids to form only alkyl halides.
a. Ethers can act as the Lewis base due to the two non-bonded electron pair on
oxygen to form coordinative bonds with Grignard reagent. This explains clearly why
organ magnesium compounds are manipulated in ether solvent but not in watersince in water, there is a reaction which generate alkanes.
b. Combustion of ethers gives carbon dioxide and water:
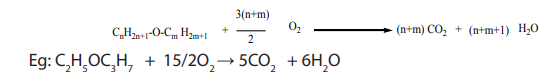
5.9.2 Oxidation reaction
Ethers react with oxygen of air to form peroxides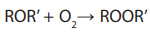
(less volatile than the parent ether)
In concentrated or solid form, these peroxides are dangerous because they are
highly explosive. The presence of peroxides contaminates the ether. This type of
contamination is purified by treatment with a reducing agent such as alkalineferrous sulphate.
5.10. Uses of ethers
Activity 5.10
A nurse is injecting anaesthesia to a patient as seen by the image below.
1. What product that form the anaesthesia.
2. Explain the effects of anaesthesia.3. In which case a patient is injected anaesthesia?
Lower ethers are used as anesthesia since they produce inert local cooling when
sprayed on a skin, ether are also used as local anesthesia for minor surgery operation.
Lower ethers are volatile liquid which on evaporation produce low temperature theyare therefore used as refrigerants.
Ether itself is one of the most important organic solvents for fats, oils, resins, andalkaloids.
Checking up 5.10:
Make a research and establish at least four uses of ethers
5.11. End unit assessment
I. Multiple choice questions
1. What is the correct name of the molecule with the skeletal formula shownbelow?
A . 1,2, 2-trimethylbutan-3-ol
B. 2-ethyl-2-methylbutan-2-ol
C. 3,3-dimethylpentan-2-olD. 4-hydroxy-3,3-dimethylpentane
2. Compound X, C4H8O2, has an unbranched carbon chain. An aqueous
solution of X has an approximate pH of 3. Compound Y, C3H8O, is a
secondary alcohol. X and Y are reacted together in the presence of
a little concentrated sulphuric acid to form Z as the major organicproduct.
What is the structural formula of Z?

3. The ester CH3CH2CH2CO2CH2CH (CH3)2 was hydrolyzed under
acidic conditions.
What are the organic products of this hydrolysis?
A. butanoic acid and 2-methylpropan-1-ol
B. butanoic acid and 2-methylpropan-2-ol
C. butan-1-ol and 2-methylpropanoic acid
D. propanoic acid and 2-methylpropan-1-ol
4. An unknown organic compound reacts with sodium to give a
combustible gas as one product but does not give a yellow precipitate
with alkaline aqueous iodine. What is a possible identity of the
unknown organic compound?
A. Propanol
B. Propan-1-olC. propan-2-ol
II. Open questions
5. A compound A is found to be soluble in sulphuric acid, A doesn’t react
is found to be soluble in sulphuric acid, A doesn’t react
with sodium or potassium permanganate. When A is heated with hydroiodic
acid, it is converted into single alkyl iodide suggest the structure of A
6. An organic compound Y possesses the centesimal composition by mass
of 87.6% carbon and the rest is hydrogen. The molecular mass of it is 56 g/
mol. Water molecule in presence of sulphuric acid was added to the same
molecule to produce M, the molecule M was subjected to sulphuric acid and
the temperatures of 140 °C, and produce N. Y possess many isomers including
cycles molecules.
Establish the structure of Y, and all isomers, M and all isomers and N. Show the
mechanism where is possible.
7. An organic liquid M contains carbon, hydrogen and oxygen. When 0.25 g of M
is combusted, 0.592g of carbon dioxide and 0.30 g of water was formed
a. i. calculate the empirical formula
ii. Molecular formula if the molecular mass is 74 g/mol
b. Write the structural formula and name of all isomers of M
c. M gives a yellow precipitate with solution of iodine in sodium hydroxide
i. Identify
ii. Describe briefly how the functional group in M may be determined
iii. Give a reaction scheme of how M can be converted into but-2-yne
8. Compare and contrast the preparation of ethanol by hydration of ethanol and
by fermentation by putting an emphasis on the advantages and disadvantagesof each process.
UNIT 6: CARBONYL COMPOUNDS: ALDEHYDES AND KETONES
Key unit competency
To be able to compare the chemical nature of carbonyl compounds to theirreactivity and uses.
Learning objectives
• Describe the reactivity of carbonyl compounds
• State the physical properties of aldehydes and ketones
• Describe the preparation reactions of ketones and aldehydes
• Explain the mechanism of nucleophilic addition reactions of carbonyl
compounds
• Prepare ketones from secondary alcohols by oxidation reactions
• Compare aldehydes and ketones by using Fehling’s solution and Tollens’
reagent
• Write and name carbonyl compounds and isomers of ketones and aldehydes
• Write equations for the reactions of carbonyl compounds with other
substances
• Compare the physical properties of carbonyl compounds to those of alcohols
and alkenes
• Differentiate the methyl ketones from other ketones by using the iodoform
test
• Carry out an experiment to distinguish between carbonyl compounds and
other organic compounds
• Carry out an experiment to distinguish between ketones and aldehydes• Carry out an experiment to prepare ethanol and propan-2-one.
6.1. Definition and nomenclature of carbonyl compounds
Introductory activity
Many fruits such as mangoes and honey contained sugar. The following imagesrepresent mangoes, honey and some sugars such as fructose and glucose.
1. State the functional groups found in fructose and glucose.
2. Enumerate other foods that contain sugars
3. Describe the similarity and difference between the two sugars in term ofstructure formulae.
6.1.1 Definition
Activity 6.1
Observe the following molecules and answer to the questions.
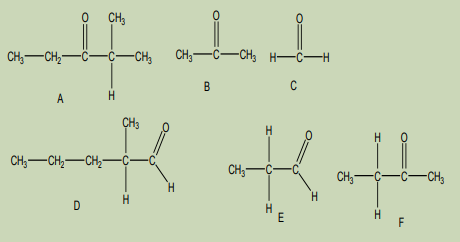
1. Categorize the above molecules
4. What criteria have you used to categorize?
5. Name those categories6. Name individual molecules
Carbonyl compounds are compounds that contain carbon-oxygen double bond
(C=O). Carbonyl compounds are classified into two general categories based on the
kinds of chemistry they undergo. In one category there are aldehydes and ketones;
in the other category there are carboxylic acids and their derivatives. This unit lookson category of aldehydes and ketones.
Aldehyde molecules
For aldehydes, the carbonyl group is attached to hydrogen atom and alkyl group as
shown in the molecule of propanal below. Methanal is the smallest aldehyde, it hastwo hydrogen atoms attached to carbonyl group.
If you are going to write this in a condensed form, you write aldehyde as –CHO,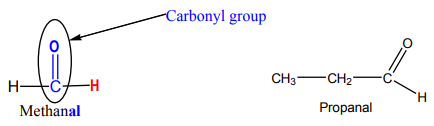
don’t write it as -COH, because that looks like an alcohol functional group.
Ketone moleculesKetone has two alkyl groups attached to the carbonyl group. Examples:

6.1.2 Nomenclature
Aldehydes
The systematic name of an aldehyde is obtained by replacing the terminal “e” from
the name of the parent hydrocarbon with “al.” In numbering the carbon chain of analdehyde, the carbonyl carbon is numbered one.

Ketones
The systematic name of a ketone is obtained by removing the terminal“e” from the
name of the parent hydrocarbon and adding “one.”The chain is numbered in the
direction that gives the carbonyl carbon the smallest number.Ketone contains
a carbon-oxygen double bond just like aldehyde, but for ketone carbonyl groupisbonded to two alkyl groups.

Checking up 6.1
7. For each of the following structures, justify whether it is an aldehyde ora ketone, and name each.
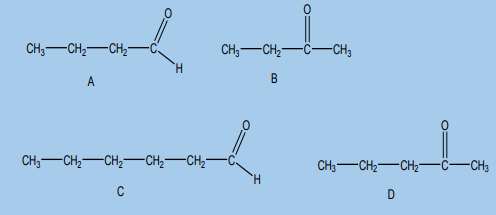
2. Draw the structural formulas derived from the following names.
i. Hexan-3-one
ii. Pentan-2-oneiii. 2-methylpropanal
6.2. Isomerism
Activity 6.2
Look at the molecules below and answer the following questions.
4. Write molecular formula of A and B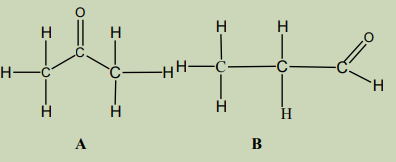
5. Compare the molecular formulae of A and B
6. State a term that can be used to describe relationship between
molecules A and B.7. Write down other three different examples which are related as A and B.
6.2.1 Functional isomerism in aldehydes and ketones
Isomers are molecules that have the same molecular formula, but have a different
arrangement of the atoms in space. Functional group isomers are molecules that
have same molecular formula but contain different functional groups, and theybelong to different homologous series of compounds.
Example1; structural formulae of this molecular formula can be either
structural formulae of this molecular formula can be either propanal or propanone, aldehyde or ketone.

You could draw others possible structural formula of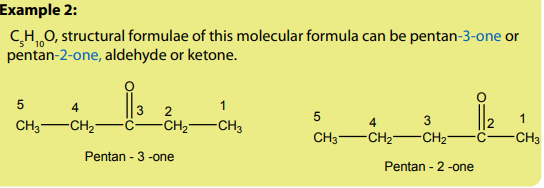

that have alkene and alcohol functional groups.
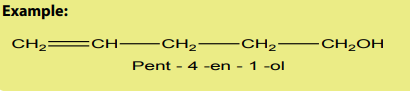
6.2.2. Position isomerism in ketones
Position isomerism is isomerism where carbon skeleton remains constant, but thefunctional group takes different positions on carbon skeleton.
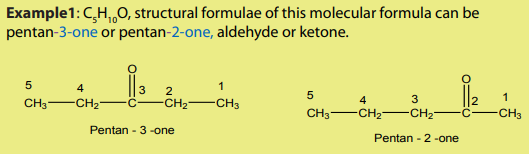
6.2.3. Chain isomerism in aldehydes and ketones
In chain isomerism the same number of carbons forms different skeletons. Aldehydes
with 4 or more carbon atoms and ketones with five or more carbon atoms showchain isomerism.
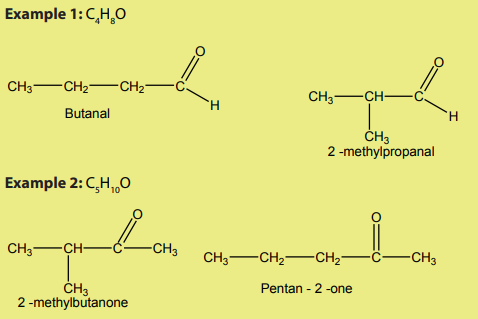
Checking up 6.2
Draw as many as possible all the structural isomers of

6.3. Physical properties of aldehydes and ketones
Activity 6.3
• Take 50 ml for each substance: ethanal, butanal and propanone.
• Mix ethanal with 50ml of water in beaker
• Mix butanal with 50ml of water in beaker
• Mix propanal with 50ml of water in beaker
viii. Compare the solubility of ethanal, butanal and propanone in water.
ix. State intermolecular forces present in each substances
x. Explain what happen in term of intermolecular forces during mixing
those above substances with water.
xi. Explain why some substances have high solubility in water than other.
xii. how the intermolecular forces present in ethanal, butanal and
propanone affect other physical properties like boiling and meltingpoint of these substances.
6.3.1. Solubility in water aldehydes and ketones
The small molecules of aldehydes and ketones are soluble in water but solubility
decreases with increase of carbon chain. Methanal, ethanal and propanone - the
common small aldehydes and ketones are soluble in water at all proportions.
Even though aldehydes and ketones don’t form hydrogen bond with themselves,they can form hydrogen bond with water molecules.
The slightly positive hydrogen atoms in a water molecule can be sufficiently attracted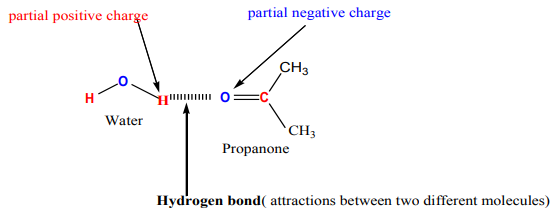
to the lone pair on the oxygen atom of an aldehyde or ketone to form a hydrogen
bond.
Other intermolecular forces present between the molecules of aldehyde or ketone
and the water are dispersion forces and dipole-dipole attractions.
Forming these attractions releases energy which helps to supply the energy needed
to separate the water molecules and aldehyde or ketone molecules from each other
before they can mix together.
Apart from the carbonyl group, hydrocarbon chains are non polar, they don’t dissolve
in water. By forcing hydrocarbon chain to mix with water molecules, they break the
relatively strong hydrogen bonds between water molecules without replacing them
by other attractions good like hydrogen bonds. This makes the process energeticallyless profitable, and so solubility decrease.
6.3.2. Boiling points of aldehydes and the ketones
Methanal is a gas and has a boiling point of
and ethanal has a boiling point of The other aldehydes and ketones
The other aldehydes and ketones
are liquids or solids, with boiling points rising with rising of molecular mass
hence rising of strength of Van der Waals force.
Comparing the physical properties of carbonyl compounds to those of alcoholsand alkanes.
Physical properties of covalent compounds depend on intermolecular forces.
Compounds that have similar molecular mass but different intermolecular forceshave different physical properties.
Example of comparison between molecules of similar mass but differentcompositions.
Alcohols have higher boiling point than aldehydes and ketones of similar lengths. In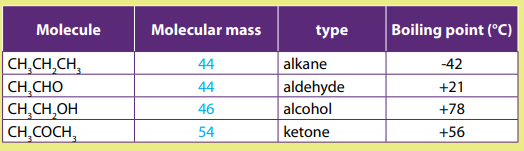
the alcohol, there is hydrogen bonding, but the molecules of aldehydes and ketones
don’t form hydrogen bonds.Aldehydes and ketones are polar molecules but alkanesare non polar molecules.
Checking up 6.3
The Table below shows the boiling points of an alkane, an aldehyde and analcohol.
m. Explain why the boiling point of an aldehyde is greater than that of the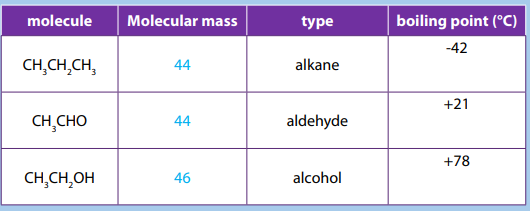
alkane?
n. Why is the boiling point of the alcohol still higher?
o. Explain why, unlike the similar-sized alkanes, the small aldehydes and
ketones are soluble in water.p. Describe the solubility variation of aldehydes and ketones.
6.4. Chemical properties of carbonyl compounds
6.4.1. Nucleophilic addition reactions
Activity 6.4.1
• KCN is a reagent used to add HCN to carbonyl compounds.
Write equation that show how KCN dissociates in polar solvent
• Observe carefully the following carbonyl functional group and answer thefollowing questions.

a. Polarity of carbonyl group
by comparing carbon-carbon double bond and carbon- oxygen double bond
the only difference between bonds C=C and C=O is distribution of electrons. The
distribution of electrons in the pi bond is heavily attracted towards the oxygen atom,because oxygen atom is much more electronegative than carbon.
During chemical reactions nucleophiles will attack carbon of the carbonyl functional
group which bears apartial positive charge. While electrophile will attack oxygen ofthe carbonyl functional group which bears a partial negative charge.
b. Reaction of HCN with aldehydes and ketones
Because hydrogen cyanide is a toxic gas, the best way to carry out this reaction is to
generate hydrogen cyanide during the reaction by adding HCl to a mixture of the
aldehyde or ketone and excess sodium cyanide. Excess sodium cyanide is used in
order to make sure that some cyanide ion is available to act as a nucleophile. The
solution will contain hydrogen cyanide (from the reaction between the sodium or
potassium cyanide and the HCl)
The pH of the solution is maintained in range 4 - 5, because this gives the fastestreaction. The reaction takes place at room temperature.
c. The mechanism of reaction between HCN and propanone
1st Step: A nucleophilic, CN-, attacks on the slightly positive charged carbon ofcarbonyl group.
2ndStep: The negative ion formed picks up a hydrogen ion from hydrogen cyanide.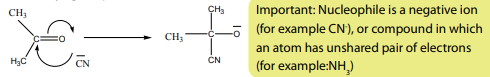
Water or the ions
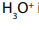 present in solution can serve as source of the hydrogen ion.
present in solution can serve as source of the hydrogen ion.
These are examples of nucleophilic addition

e. Reaction of
The aldehyde or ketone is shaken with a saturated solution of sodium hydrogen with aldehydes or ketones
with aldehydes or ketones
sulphite in water. Hydrogen sulphite with negative charge act as nucleophile,
where the product formed is separated as white crystals. Propanone react hydrogensulphite, as below:
Impure aldehyde and ketone can be purified by using this reaction. Impure
aldehyde or ketone is shaken with a saturated solution of sodium hydrogensulphite
to produce the crystals. Impurities don’t form crystals; these crystals formed are
filtered and washed to remove any impurities. Addition of dilute acid to filtered
crystals regenerates the original aldehyde. Dilute alkali also can be added insteaddilute acid.
Checking up 6.4.1
Aldehydes and ketones undergo addition reactions involving hydrogen
cyanide in which H and CN add on the carbon-oxygen double bond.
a. Why isn’t hydrogen cyanide itself normally used in these reactions?
b. Give a mixture which can be used instead of starting with hydrogen
cyanide itself.
c. Draw the structures and give the names of the products of the
reaction between hydrogen cyanide and
i. (Ethanal
ii. Propanone
d. One use of the products of these reactions (known as hydroxy nitriles)
is as a part of a sequence of reactions to make more complicated
molecules like amino acids from more simple ones. The amino acidvaline has the structure:
i. Write the structure of the hydroxy nitrile which you would have to modify
in order to make valine
ii. Write the structure of the aldehyde or ketone which you would have toreact with hydrogen cyanide in order to get that hydroxy nitrile
6.4.2. Condensation reactions
Activity.6.4.2
You are provided with the following: propanal, propanone, alcohol (ethanol),
glucose solution and 2,4-dinitrophenylhydrazine (Brady reagent )
Take about 2ml of each solution; propanal, propanone, alcohol (ethanol) and
glucose solution in test tubes. Add 6 drops of the 2,4-dinitrophenylhydrazine
to each of the test tubes containing: propanal, propanone, (alcohol)ethanol or
glucose solution. If no precipitate forms immediately, warm for 5 minutes in thewater bath. Record your observations in the table below.
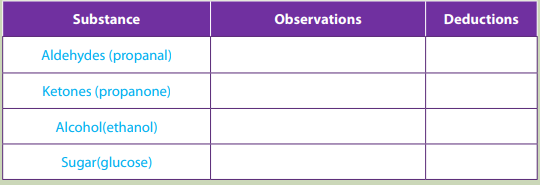
a. Experimental reaction
The procedure of the preparation of Brady’s reagent and carbonyl compounds
changesslightly depending on the nature of the aldehyde or ketone, and the solvent
in which 2,4-dinitrophenylhydrazine is dissolved in. The Brady’s reagent for activities(6.4.1) is a solution of the 2,4-dinitrophenylhydrazine in methanol and sulphuric acid.
Add a few drops of Brady’s reagent to either aldehyde or ketone. A bright orange or
yellow precipitate indicates the presence of the carbonyl group in an aldehyde orketone.
b. Structural formula of 2,4-dinitrophenylhydrazine.
The carbon of benzene attached to hydrazine is counted as number one.
In 2,4-dinitrophenylhydrazine, there are two nitro groups,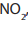
,attached to the phenyl group in the 2- and 4- positions.
2,4-dinitrophenylhydrazine is often abbreviated as 2,4-DNP or 2,4-DNPH.
c. The reaction of carbonyl compounds with 2,4-dinitrophenylhydrazine
Brady’s reagent is a solution of the 2,4-dinitrophenylhydrazine in methanol
and sulphuric acid. The overall reaction of carbonyl compounds with2,4-dinitrophenylhydrazine is:
Where R and R’ represent alkyl groups or hydrogen(s); if both or only one is hydrogens
the starting carbonyl compound is an aldehyde. If both R and R’ are alkyl groups
the carbonyl compound is a ketone. The following molecule shows clearly how theproduct is formed.
The product formed is named”2,4-dinitrophenylhydrazone”. The simple difference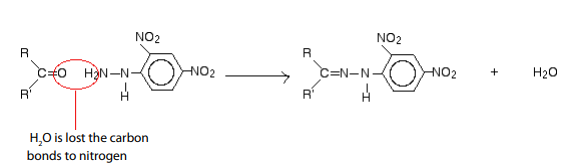
consists in replacing suffix “-ine” by “-one”.
The reaction of 2,4-dinitrophenylhydrazine with ethanal produces ethanal
2,4-dinitrophenylhydrazone; The reaction of 2,4-dinitrophenylhydrazine with
butanal produces butanal 2,4-dinitrophenylhydrazone. This is an example ofcondensation reaction.
During the chemical reaction, the change takes place only on nitrogen of
of
hydrazine in 2,4-dinitrophenylhydrazine. If the group is attached to other
group is attached to other groups a similar reaction as that of 2,4-dinitrophenylhydrazine will take place:
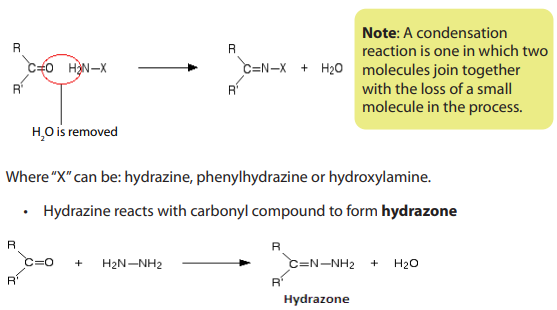

• Phenylhydrazine reacts with carbonyl compound to form“phenylhydrazone”.
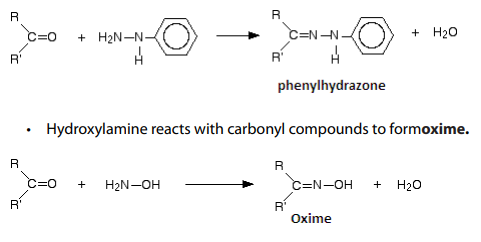
Checking up 6.4.2
a. Brady’s reagent is a solution of 2,4-dinitrophenylhydrazine in a mixture
of methanol and sulphuric acid.
i. How is Brady’s reagent used to test for an aldehyde or ketone?
b. Draw the structural formulae for
ii. Propanone hydra zone
iii. Propanone phenylhydrazone
6.4.3. Oxidation reactions using
Activity 6.4.3
Materials:
• Test tubes
• Test tubes holder
• Test tube racks
• Count droppers
• Beakers
You are provided with the following: propanal, propanone and potassium dichromate
(VI) solution acidified with dilute sulphuric acid.
Take about 2ml of each solution; propanal and propanone; add 6 drops of the
potassiumdichromate(VI) solution acidified with dilute sulphuric acid.
Record your observations in the table below.
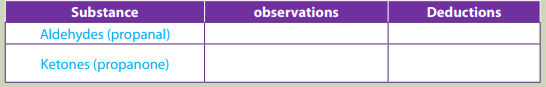
a. Difference in reactivity of ketones and aldehydes with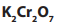
By considering the structural formulae of aldehydes and ketones, the difference is
only the presence of a hydrogen atom attached to the carbonyl functional group inthe aldehyde whereas ketones have a alkyl group instead.
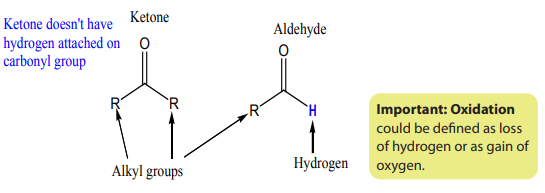
During chemical reaction aldehydes react with oxidizing agent; hydrogen on
carbonyl functional group is replaced by oxygen, look on figure below. The presence
of hydrogen atom makes aldehydes very easy to oxidize, in other words aldehydesare strong reducing agents.
For ketone, absence of hydrogen on carbonyl functional group makes ketones to
resist oxidation. But very strong oxidising agents like potassium permanganate
solution oxidize ketones - and they do it in a destructive way, by breaking carbon bonds.
Aldehyde oxidation can take place in acidic or alkaline solutions. Under acidic
solutions, the aldehyde is oxidized to a carboxylic acid. Under alkaline solutions, acidformed react with base to form a salt of carboxylic acid.
Add few drops of the aldehyde or ketone to a solution of potassium dichromate
(VI) acidified with dilute sulphuric acid. If the color doesn’t change in the cold, themixture is warmed gently in a beaker containing hot water.

b. Oxidation of aldehyde by
Add few drops of the aldehyde or ketone to a solution of potassium dichromate solution
solution
(VI) acidified with dilute sulphuric acid. If the color doesn’t change in the cold, themixture is warmed gently in a beaker containing hot water.

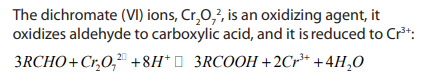
Checking up 6.4.3
1. If you react ethanal with acidified potassium dichromate (VI) solution,
what organic product would you get?
2. Write a half-equation for the formation of that product from ethanal.
3. iii.Write a half-equation for the dichromate(VI) ion acting as an oxidisingagent is
Use this equation and the one you wrote in part (ii) to work out the ionic
equation for the reaction.
6.4.4. Oxidation reactions using Tollens’ reagent
Activity 6.4.4
Materials:
• Test tubes
• Test tubes holder
• Test tube racks
• Count droppers
• Beakers
• Bunsen burner
You are provided with the following:
• propanal,
• propanone
• Tollens’ reagent.
Take about 2ml of each solution; propanal and propanone. Add 6 drops of
the Tollens’ reagent to each of the following in the test tubes; propanal or
propanone. Warm gently the mixture in a hot water bath for a few minutes.Record your observations in the table below.
a. Difference in reactivity of Ketones and Aldehydes with Tollens’ reagent
Aldehydes can also be oxidized into carboxylic ions in basic medium.Tollens’ reagent
is a solution of diamminesilver (I) ion,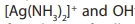 .In order to identify if a
.In order to identify if a
substance is aldehyde or ketone, add few drops of Tollens reagent to test tubes
containing aldehyde or ketone and warm gently in a hot water bath for a few
minutes. The formations of sliver mirror or grey precipitate is an indication of thepresence of aldehyde.

Refer to experimental student book:
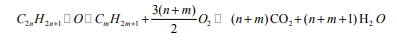
Checking up 6.4.4
1. Tollens’ reagent is alkaline because of the sodium hydroxide solution
and ammonia solution used to make it. What organic product would you
get in this case if you reacted propanal with Tollens’ reagent?
2. Write half equation for the formation of that product from propanal.
3. Write the half-equation for the reaction of the ion when it
ion when it
forms the visible product of the reaction.
Combine these two half-equations to give an ionic equation for the reaction ofTollens’ reagent with ethanal.
6.4.5. Oxidation reactions using Fehling ;or Benedict; solution
Activity 6.4.5
Materials:
• Test tubes
• Test tubes holder
• Test tube racks
• Count droppers
• Beakers
• Bunsen burner
You are provided with the following: ethanal, propanone, Fehling’s solution and
Benedict’s solution.
Take about 2ml of each solution. Add 6 drops of the Fehling’s solution
or Benedict’s solution to each of the tubes containing 2ml of ethanal or
propanone to be tested. Warm gently the mixture in a hot water bath for a fewminutes. Record your observations in the table below.
a. Difference in reactivity of Ketones and Aldehydes with Fehling or Benedict
solution.
Fehling’s solution and Benedict’s solution react with aldehyde in the same way;
both solutions contain . In Fehling’s solution
. In Fehling’s solution  is complexed with tartrate
is complexed with tartrate
ligand butin Benedict’s solution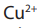 is complexed with citrate ligand.
is complexed with citrate ligand.
Don’t worry about ligands, important reagents are ligands tartrate and
ligands tartrate and
citrate are used to prevent formation of precipitate copper (II) hydroxide or copper
(II) carbonate.
A few drops of Fehling’s solution or Benedict’s solution is added to the aldehyde orketone and the mixture is warmed gently in a hot water bath for a few minutes.
Fehling’s solution and Benedict’s solution are oxidizing agent, they oxidize aldehydes
to carboxylic acid. Remember that reaction takes place in basic solutions, acid
formed is neutralized by base, and hence the products area salt of carboxylic acidinstead of carboxylic acid. Equations of reaction.

Checking up 6.4.5
Fehling’s solution and Benedict’s solution both contain copper (II) complexes
in an alkaline solution. The copper (II) complex can be simplified to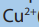 (in
(in complex), and the electron-half-equation given as
a. Write the electron-half-equation for the oxidation of propanal in an

alkaline solution.
b. Combine this with the equation above to give the ionic equation for thereaction between Fehling’s or Benedict’s solution with propanal
6.4.6. Iodoform reaction with aldehydes and ketones
Activity 6.4.6
Materials:
• Test tubes
• Test tubes holder
• Test tube racks
• Count droppers
• Beakers
• Bunsen burner
You are provided with the following: propanone, propanal, 6M NaOH solution
and solution
solution
Put 4 drops of each tested substances, propanone, propanal, into different test
tubes.
Add to this 0.5 mL distilled water to each test tube.
Add 0.25mL 6M NaOH and 0.25 mL of water to each test tube.
Add 6 drops of -s solution to each test tube.
solution to each test tube.
If no precipitate forms immediately, warm the mixture very gently.Record your observations in the table below.
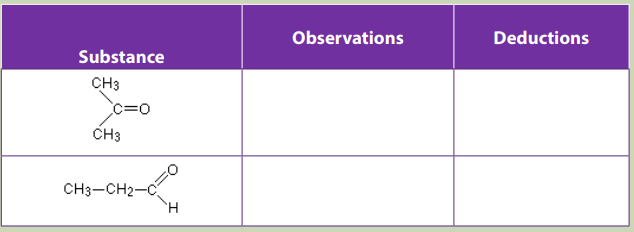
a. Reagents for iodoform reaction
There are two different mixtures that can be used to do iodoform test, these
mixture are:
• Iodine and sodium hydroxide solution
• Potassium iodide and sodium chlorate (I) solutions
Don’t worry about Potassium iodide and sodium chlorate(I) solutions, Potassium
iodide and sodium chlorate(I) react to form final solution
.Both mixtures contain the same reagents.
Each of these mixtures contains important reagent which react
which react
with aldehyde or ketone. When is added to a carbonyl compound
is added to a carbonyl compound
containing the group (blue in the cycle) as shown below, pale yellow precipitate
(blue in the cycle) as shown below, pale yellow precipitate (triiodomethane) is formed.

a. Description of iodoform test
For iodine and sodium hydroxide solution
Iodine solution,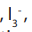 is added to aldehyde or ketone, followed by just enough sodium
is added to aldehyde or ketone, followed by just enough sodium hydroxide solution to remove the colour of the iodine. If pale yellow precipitate
doesn’t form in the cold, it may be necessary to warm the mixture very gently.
The positive result is pale yellow precipitate of
For potassium iodide and sodium chlorate (I) solutions
Potassium iodide solution is added to a small amount of aldehyde or ketone, followed
by sodium chlorate (I) solution. If pale yellow precipitate doesn’t form in the cold,warm the mixture very gently. The positive result is pale yellow precipitate of
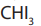
Reaction of iodoform test
The reagents of iodoform test are and OH solution. The reaction takes place into
and OH solution. The reaction takes place into
two main steps:
• Three hydroxides, OH-, remove three hydrogens from methyl group and theplace of hydrogen is taken by iodide
• group
 is a good leaving group;
is a good leaving group;  is replaced by OH-to form carboxylic
is replaced by OH-to form carboxylic
acid, because is a base according to Bronsted-Lowry, it reacts with acid to
is a base according to Bronsted-Lowry, it reacts with acid to form the following product:
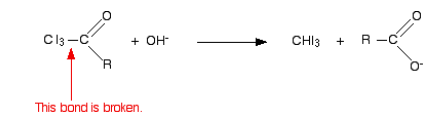
The overall equation for reaction of iodoform test:
The same reaction takes place for other halogen elements in the same way.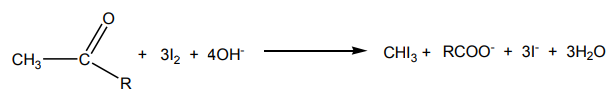
Refer to experimental student book.
When methyl ketones or methyl aldehyde, ethanal, are treated with the halogen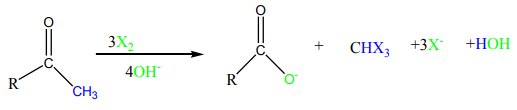
element in basic solution, hydrogens of the methyl group are replaced by halogen
element followed by cleavage of the methyl group. The products are the salt of
carboxylic acid and trihalomethane. The reaction is fast until the 3 hydrogens at themethyl group have been replaced by a halogen.
Checking up 6.4.6
A has the formula Its oxidation gives B with the formula
Its oxidation gives B with the formula 
B reacts with 2,4-dinitrophenylhydrazine to give a positive test. A is dehydrated by
concentrated . Reductive of C gives butanal.
of C gives butanal.Identify the compound A
6.5. Preparation methods of aldehydes and ketones
6.5.1. Oxidation of alcohols
Activity 6.5.1
The set up below represents the method of preparation of ethanal from ethanol.Look it carefully and answer the following questions.
Ethanol reacts with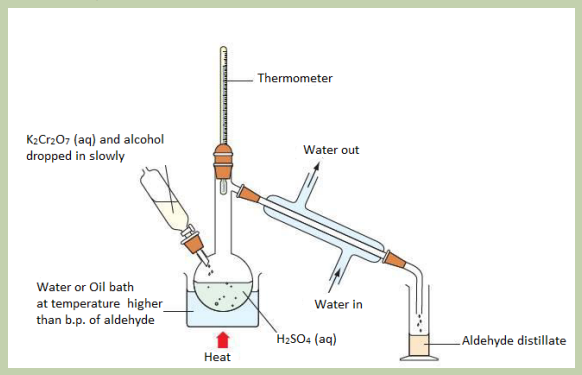
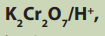 does ethanol undergoes oxidation or
does ethanol undergoes oxidation or
reduction in this reaction?
3. Write down chemical equation that takes place in this experiment
4. Explain why it is necessary to heat and explain the point of choosing
temperature at which reaction takes place.5. Write a balanced equation of the reaction between propan-2-ol and
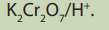
a. alcohol by
Pdichromate (VI) ions is reduced to chromium (III) ions, Cr3+ which is green.otassium dichromate (VI) acidified with dilute sulphuric acid is used as oxidizing
agent during the preparation of aldehyde or ketone. Primary alcohol is oxidized to
aldehyde, oxygen atom from the oxidising agent removes two hydrogens; one from
the -OH group of the alcohol and the other hydrogen comes from the carbon that isattached to hydroxide functional group.
• Primary alcohol undergoes oxidation to produce aldehyde

• Secondary alcohol undergoes oxidation to produce ketone
• Tertiary alcohol doesn’t undergo oxidation because the carbon bonded to
hydroxide doesn’t have hydrogen to be removed.
The solution of dichromate (VI) ions,
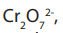 , is orange, during chemical reaction
, is orange, during chemical reactiondichromate (VI) ions is reduced to chromium (III) ions,
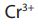 which is green.
which is green.
b. Technique of stopping oxidation of aldehyde
The aldehyde produced by oxidation of alcohol could make further oxidation to a
carboxylic acid if the acidified potassium dichromate (VI) is still present in solution
where reaction takes place. In order to prevent this further oxidation of aldehyde to
carboxylic the following technique are used.
• Use an excess of the alcohol than potassium dichromate (VI). Potassium
dichromate (VI) is limiting reactant hence there isn’t enough oxidising agent
present to carry out the second stage of oxidizing the aldehyde formed to a
carboxylic acid.
• Distil off the aldehyde as soon as it forms. Removing the aldehyde as soon as it
is formed this means that aldehyde is removed from solution where oxidizing
agent is, to prevent further oxidation. Ethanol produces ethanal as shown bythe following reaction.
To simplify the writing of the reaction, [O] represents oxygen from an oxidising
agent. Then the reaction is written as follows:
1. If you want to oxidize ethanol to ethanal without further oxidation to
ethanoic acid, how do you proceed?
2. Which oxidising agent is used to oxidize alcohols to either aldehydes orketones, and what would you observe during the reaction?
Checking up 6.5
1. Draw the structure of the aldehyde or ketone that would be formed
if each of the following alcohols is oxidised. You can assume that
conditions are fulfilled to avoid further oxidation of the aldehyde to acarboxylic acid.
2. Draw the structure of the alcohol you would oxidize in order to obtain
each of the following compounds.
i. pentan-2-oneii. Butanal
c. Oxidation of alkene by
Oxidation of alkenes with hot concentrated acidified potassium manganate (VII)
solution produces carbonyl compounds. Consider the general formula of alkenebelow:
Where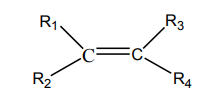
 represent alkyl groups or hydrogen atoms
represent alkyl groups or hydrogen atoms
Carbon-carbon double bond of alkene is broken by acidified potassium manganate
(VII) and is replaced by two carbon-oxygen double bonds to each carbon fromdouble bond. General equation:
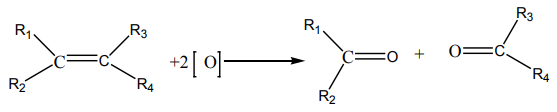
If acidified potassium manganate (VII) is still present in solution, aldehyde makes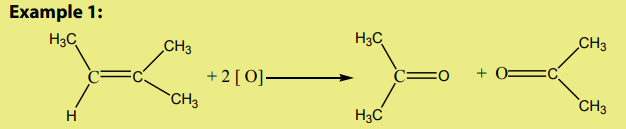
further oxidation to carboxylic acid.
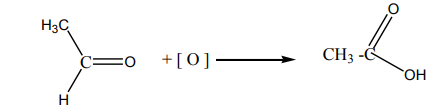
Methanoic acid has hydrogen attached on carbonyl group hence it makes further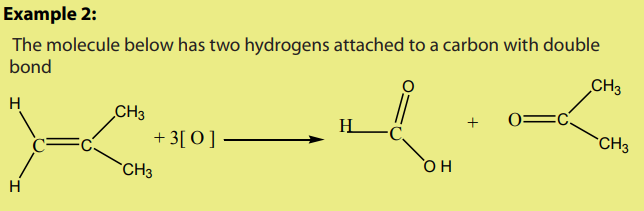
oxidation to carbon dioxide. Final equation is written as below:
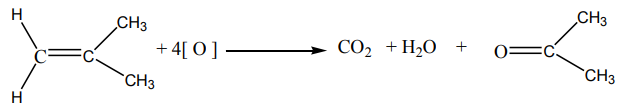
6.5.2. Preparation of ketone by distillation of calcium acetate
Procedure: Transfer 15g of calcium acetate in 50ml round bottom flask fixed on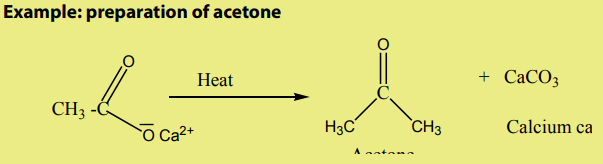
a stand, and place it on a heating mantle fitted with a condenser and a receiver
flask. Adjust the temperature until the condensation starts. Use the aluminium
foil to insulate the flask. Heat the flask and collect the acetone in receiver flask. The
obtained product is a crude acetone and needs to be purified. Set up a distillation
apparatus and distil the crude product to obtain pure acetone Do not forget
Do not forget
to use stirrer bar which must be placed in the round bottom flask containing theacetone.

6.6. Uses of aldehydes and ketones
Aldehydes and ketones have many uses for example in industries such aspharmaceutical industry and in medicine.
a. Formaldehyde:
Formaldehyde is a gas at room temperature but is sold as a 37 percent solution in water.
Formaldehyde is used as preservative and germicide, fungicide, and insecticide
for plants and vegetables. Formaldehyde is mainly used in production of certain
polymers like Bakelite (Figure 6.1). Bakelite and formaldehyde is used asmonomers in production of Bakelite.
b. Acetone as solvent:
Acetone is soluble in water at all proportions and also dissolves in many organic
compounds. Boiling point of acetone is low, 56 °C, which makes it easier to be removed by
evaporation. Acetone is an industrial solvent that is used in products such as paints,
varnishes, resins, coatings, and nail polish removers.
c. Aldehydes and ketones
Organic molecules that contain ketones or aldehydes functional group are found in
different foods such as irish potatoes, yellow bananas.
Aldehydes and ketones perform essential functions in humans and other living organisms.
For examples sugars, starch, and cellulose, which are formed from simple molecules that
have aldehyde or ketone functional group.
d. Aldehydes and ketones in human’s body

Aldehydes and ketones functional group are found in humans hormones likeprogesterone, testosterone.
6.7. End Unit Assessment
1. An aliphatic aldehyde A has the formula RCHO.
a. A reacts with 2,4-dinitrophenylhydrazine. Explain what happens and
name the type of reaction.Say how the product of reaction could be used to identify A
b. When A is treated with warm, acidified solution, B is formed.
solution, B is formed.
Give the structural formula of B.
c. When A is treated with lithium tetrahydridoaluminate (reducing agent)
in ethoxyethane solution C is formed. Give the structural formula of C.
d. A is warmed gently with ammoniacal silver nitrate. Explain what
happens, and say what is observed.
e. B and C react to form D. Write the structural formula of D.
f. From the compounds A, B, C, and D, which would you predict to
possess:
i. Highest boiling pointii. Lowest boiling point
2. Three compounds E, F, and G all have the molecular formula
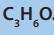
E is an alcohol, F is ketone and G is aldehyde.
i. Draw all possible structural formulae for E, F, and G.
ii. Describe tests (reagents, conditions and observations with each
compound) that would allow you to show that.
3. E is an alcohol whereas F and G aren’t
4. F and G are carbonyl compounds whereas E isn’t
5. G is aldehyde, whereas E and F aren’t.
6. Write balanced equations for all reactions that occur.
a. One of the compounds responsible for the flavor of butter is butan-2,3-
dione.
i. Give the structural formula of butan-2,3-dione.
Give the structural formula of the organic products formed when butan-2,3-dione reacts with

3. Carbonyl compounds X undergoes the following reactions
X gives an orange precipitate with 2, 4-dinitrophenylhydrazine.
X gives pale yellow precipitate with mixture of potassium iodide and sodium
iodate (I) X Doesn’t react with warm acidified
X doesn’t react with aqueous bromine.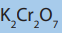 solution.
solution.
X is reduced by hydrogen in the presence of catalyst to a mixture of isomers
Y and Z of formula Identify X, and give the structural formulae of X, Y
Identify X, and give the structural formulae of X, Yand Z.
4. P has the formula . It forms a compound by reaction with hydrogen
. It forms a compound by reaction with hydrogen
cyanide which has the formula . P gives a positive iodoform test, a
. P gives a positive iodoform test, a
silver mirror with Tollens’ reagent and can be reduced to pentane. What is P?
5. a. The carbon-oxygen double bond present in aldehydes and ketones is very
polar. What does this mean and how does it arise?
b. The carbon-oxygen double bond is readily attacked by nucleophiles like
cyanide ions or ammonia.
i. What do you understand by the term nucleophile?
ii. Which part of the carbon-oxygen double bond is attractive to
nucleophiles?
6. Warfarin is an oral anticoagulant, a drug that inhibits the clotting of blood.
It prevents the formation of blood clots by reducing the production of factors
by the liver that promote clotting, factors II, VII, IX, and X, and the anticoagulantproteins C and S. The structural formula of Warfarin is:
a. Name any three different functional groups present in the Warfarin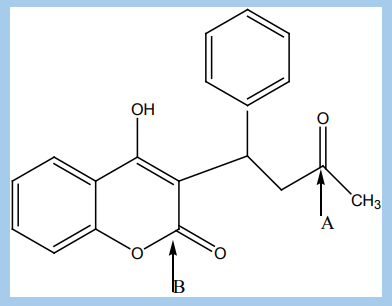
molecule.
UNIT 8: ESTERS, ACID ANHYDRIDES, AMIDES AND NITRILES.
Key unit competency:
To be able to relate the functional groups of esters, acid anhydrides,
amides and nitriles to their reactivity, preparation methods and uses.
• Describe the chemical properties of esters, acid anhydrides, amides, and
nitriles.
• Describe the process of urea manufacture and its uses.• Describe the formation of the detergents.
Apply IUPAC rules to name esters, acid anhydrides, amides, and nitriles.
• Compare the physical properties of esters to those of alcohols and carboxylic
acids.
• Make a soap and compare its properties with those of soapless detergents.
• Compare the reactivity of acid anhydrides with those of acyl chlorides.
• Prepare aspirin from appropriate reagents.
• Appreciate the importance of esters, acid anhydrides, amides and nitriles intextile industry and pharmacy.
Introductory activity
The development of organic chemistry has led scientists to the production of
new substances and materials that are necessary in our everyday life which
could not be provided by our natural environment. Others were produced to
satisfy the high demand of consumers which cannot be assured by natural
products only. Analyze the items presented below and answer the questionslisted down.

1. What kind of textile is used to make umbrellas? How did scientists make
this kind of textiles?
2. One of the substances used to improve soil fertility so as to ensure food
security is urea? How is it synthesized?
3. Why is it possible to make artificial drinks with flavors of natural fruits?
4. How are pain killer drugs manufactured?5. What kinds of substances provide perfumes with their fragrances?
8.1. Structure and nomenclature of esters
Activity 8.1
3. The compounds listed below contain acid derivatives and other organicmolecules. Classify them in the following table.
2.Draw all possible isomers with molecular formula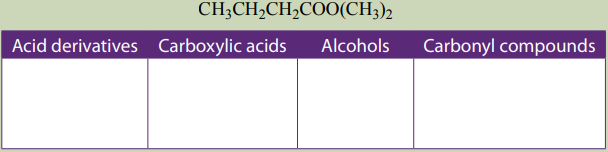
 and label esters
and label esters with letter A and acids with letter B.
8.1.1. Structure of esters
In unit 7, the reactions of carboxylic acids were discussed. The reactions of carboxylic
acids produce the derivatives of acids such as esters, acid halides, acid anhydridesand amides.
The general molecular formula of esters is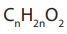 and their general structural
and their general structural formula is: RCOOR’ or
Where R may be a hydrogen atom or an alkyl group and R may be an alkyl group
or an aryl group but not a hydrogen atom. In case that R is the hydrogen atom, the
compound is no longer an ester but it is a carboxylic acid.
The following Figures, 8.1 and 8.2 show models for two common esters where green
spheres = Hydrogen atoms, red spheres = oxygen atoms; blue spheres = carbonatoms.
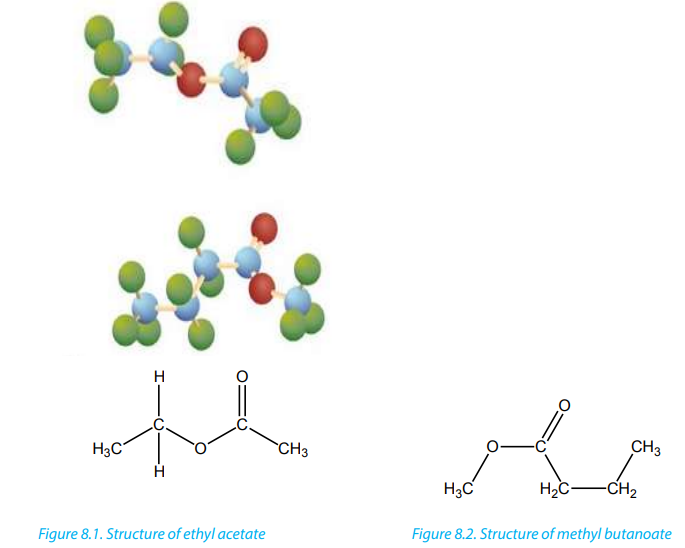
The functional group of esters is
Esters are compounds produced by the reaction involving an acid and an alcohol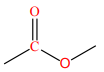
with the elimination of water molecule.
For example, the reaction between acetic acid and ethanol yields an esterwith water.
Esters are known for their distinctive odor and they are commonly responsible for
the characteristic of food (fruits) aroma, flowers and fragrances. Esters are found in
nature but they can be also synthesized. Both natural and synthetic esters are usedin perfumes and as flavoring agents.
8.1.2. Nomenclature of esters
The nomenclature of esters follows some steps. When naming esters the alkyl
group R’ is named followed by the name of RCOO- group.
The group name of the alkyl or aryl portion is written first and is followed by the
name of the acid portion. In both common and International Union of Pure and
Applied Chemistry (IUPAC) nomenclature, the -ic ending of the corresponding acid
is replaced by the suffix –ate. Some examples of names of esters are given in Table8.1.
Examples:
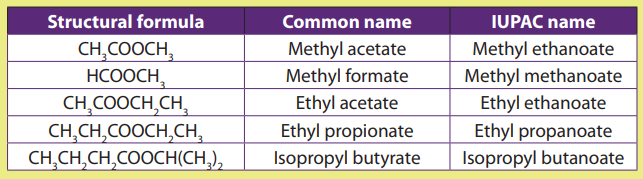
Table 8.1. Examples of structural formulae of some esters and their name
8.1.3. Physical properties and uses of Esters
Experiment
A. Analyzing the solubility of esters ( fats and oils)
Materials and Chemicals
Cooking oil, margarine, water, ethanol, stirring rods and test tubes labeled A
and B.
Procedure
1. Pour water in a test tube A and ethanol in test tube B and add some
cooking oil in each test tube. Shake well to mix and record your
observation.
2. Pour water in a test tube A and ethanol in test tube B and add a small
piece of margarine in each test tube. Use a stirring rod to mix and record
your observation.
Conclusion: Esters are soluble in organic solvents such as ethanol and insolublein water.
B. Comparing boiling points of alcohols, carboxylic acids and esters
Materials and Chemicals
Propan-1-ol, propanoic acid and methyl ethanoate, test tubes, test tube holders
(lacks), heaters, and thermometers.
Procedure
1. Put 10 mL of each substance in a labeled test tube.
2. Boil carefully substances are volatile and flammable
3. Use a thermometer to measure the boiling point of each substance.
4. Record the results and compare them. Suggest a reason for the difference
in boiling points of the three substances.
Conclusion: Esters have lower boiling points than alcohols and carboxylic
acids because they lack hydrogen bonds. A compound having hydrogen bonds
has a high boiling point because, to break that bond requires higher energy.
Other physical properties of esters
i. Lower esters have sweet fruity smells
ii. Melting and boiling points of esters increase as the molecular mass
increases.
iii. Small esters are fairly soluble in water but the solubility decreases as the
length of the chain increases
8.1.4. Uses of Esters
Esters find various uses:
i. They are used as organic solvent
ii. Due to their aroma, they are used as constituent of fragrance, essential oils,
food flavoring and cosmetics.
iii. They are used to manufacture soaps, detergents and glycerol.
iv. They are used to provide energy in the bodyv. Polyesters are used to produce plastics etc
Checking up 8.1
1. Name the following compounds by using the common and IUPACnames.
2. Draw the structural formulae corresponding to each of the following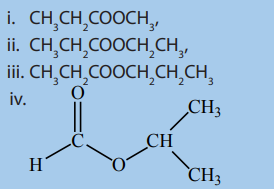
names.
i. Propyl methanoate
ii. octyl ethanoate
3. Discuss the solubility of esters
4.State one industrial and one biological use of esters.
5.Two compounds A and B of molecular formula were analyzed
were analyzed
to compare their relative boiling points. Compound A had lower boiling
point than compound B. Draw the structural formula of each compound.
6.Identify the relationship that exists between compounds A and B. Explainyour reasoning.
8.2. Preparation and chemical properties of esters
3. Using your research on internet and reading books, make a summary of
each of the following terms:
e. Reduction of esters
f. Hydrolysis of esters
g. Alkaline hydrolysis of ester
h. Trans-Esterification
i. Comparison of the reactivity of esters, acid chlorides and acid
anhydrides
4.Give an example of an equation for each of the processes in (1).5. Express the technical name given to the process in 3 (c)
8.2.1. Preparation of Esters
The preparation of esters involves different types of reaction such as esterification,
reaction of an acid chloride with an alcohol and the reaction of acid anhydrides
with alcohols.
1.Esterification reaction
In units five and seven, it is mentioned that esters can be produced by a reaction
between alcohols and carboxylic acids in strong acidic medium acting as a catalyst.
The acid is commonly a concentrated sulphuric acid, under reflux (Figure 8.3). The
reaction is generally called “Esterification” (a condensation reaction which involves
the addition of the alcohol and acid molecules followed by an elimination of awater molecule).

3. Reaction of acid anhydrides with alcohols ( Trans-esterification)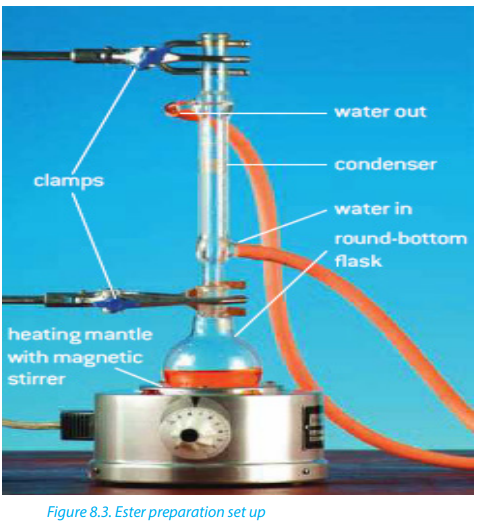
Alcohols react with esters to undergo an exchange of the alkoxide segment. The
reaction is acid catalyzed and the used alcohol must be in excess. This is a verycommon technique of producing new esters from available esters.


Reaction mechanism

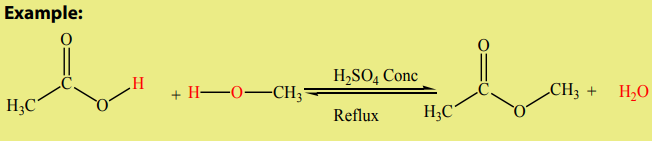
2. Reaction of an acid chloride with an alcohol
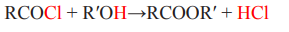

8.2.2. Chemical properties of esters
Chemical properties of esters involve their reactivity with other compounds.
a. Reduction of esters
Compared to ketones and aldehydes, esters are relatively resistant to reduction.
Esters are reduced by giving two alcohols,
giving two alcohols,
one from the acyl segment (RC=O) and one from the alkoxide segment (R-O) asshown by the reaction below.
When a less reactive reducing agent such as diisobutylaluminium hydride (DIBAH) is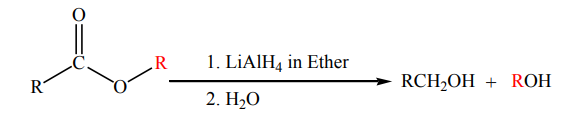
used the acyl segment is converted into an aldehyde and the alkoxide group is still
converted into an alcohol. Exactly one equivalent of the hydride must be used, andthe reaction must be carried out at -78 °C
b. Hydrolysis of esters.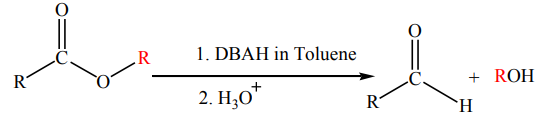
The reaction of an ester with water is called hydrolysis. This reaction is very slowunless catalyzed by a base or an acid.

Mechanism of basic hydrolysis ofeEsters
The base catalyzed hydrolysis reaction is called saponification (derived from Latin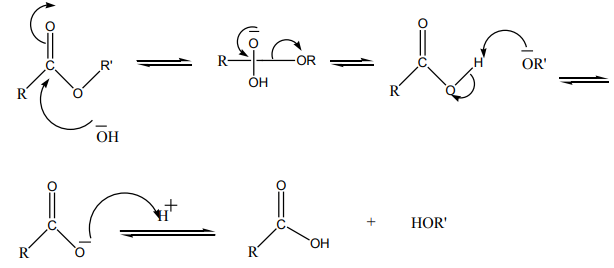
word, “sapo”, which means soap). Soaps are sodium or potassium salts made by
hydrolyzing the vegetable oil which contain higher molecular weight esters in thepresence of sodium or potassium hydroxides.
c. Trans-esterification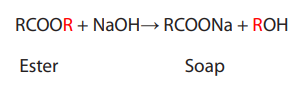
Alcohols react with esters to undergo an exchange of the alkoxide segment. The
reaction is acid catalyzed and the used alcohol must be in excess. This is a verycommon way of producing new esters from readily available esters.
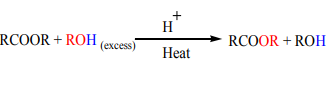
d. Reaction with amines: Aminolysis
Esters react with ammonia, primary or secondary amines to produce amides. The
reaction is carried out at high temperature in basic medium. However, this reaction isnot often used because higher yields are normally obtained by using acyl chlorides.
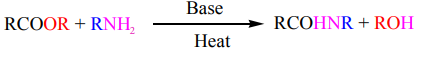
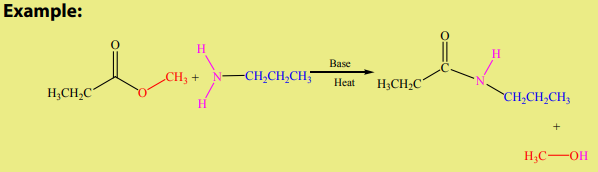
e. Reaction with Grignard reagents
Esters react with two equivalents of a Grignard reagent to form tertiary alcohols. This
reaction produces ketone intermediates which undergo a fast conversion into thealcohol because of being more reactive than esters.
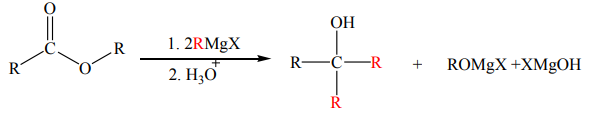
Checking Up 8.2.
1. Write a balanced equation for the reaction between propanoyl
chloride and butan-1-ol and name the product.
2. Investigate how to carry out the following conversions by using a
non-organic compounds other than the one cited. Use any inorganic
substances you need.
a. Propan-1-ol to propyl propanoate
b. Ethanal to ethyl ethanoate
3. Ethanoic acid reacts with an alcohol of molecular formula C4H10O to
produce an ester which is optically active.
a. Identify the structure of the alcohol.b. Sketch the structure of the ester formed.
4. Complete the equations below:
5. For a reaction to take place, some conditions may be required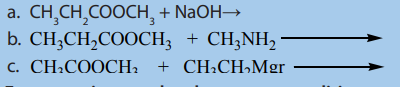
depending on the type of reaction. Discuss the conditions to be usedin order to carry out the reaction 4.a.
6. Reactions of amines with esters are not common
Explain briefly this statement.
7. You are provided with ethyl ethanoate and asked to prepare isobutyl
ethanoate.
Describe how you can proceed to prepare that compound. In your
explanations, include reagents, conditions and equation(s) for the reaction(s)
that take place.You are allowed to use any other organic compound you need.
8.3. Saponification and Detergents

Observe the above picture and answer the following questions
1. Explain the properties that these products have which make them
suitable for their use as you have stated in (1).
2. Explain how these products are manufactured?
3. Propose the differences and similarities of these products?
4. Using NaOH and cooking oil, how can you prepare a solid soap inlaboratory?
Surfactants like soaps and detergents are important cleaning products which play
an essential role in our daily life. By safely and effectively removing soils, germs and
other contaminants, they help us to stay healthy, care for our homes and possessions,
and make our surroundings more pleasant.
Soaps
Soaps are water-soluble sodium or potassium salts of fatty acids. Soaps are made
from fats and oils, or their fatty acids, by reacting them with a strong alkali. The
process is known as “saponification”.
Fats and oils
The fats (solid lipids at room temperature and pressure) and oils (liquid lipids at
room temperature and pressure) used to produce soaps find their sources from
animal or plant. Each fat or oil is made up of a distinctive mixture of several different
triglycerides.
In the formation of a triglyceride molecule, three fatty acid molecules reacted withone molecule of propane-1,2,3-triol or glycerol as shown in Figure 8. 4 below.
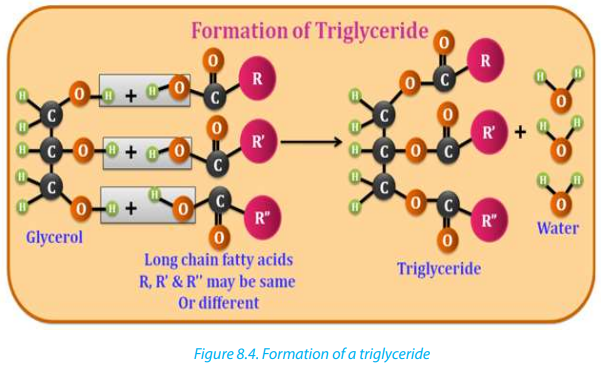

Saponification reaction
The reaction of saponification involves the collision between triglycerides in fat/oiland aqueous NaOH or KOH. The result is the formation of soap and glycerol (Figure 8.5).
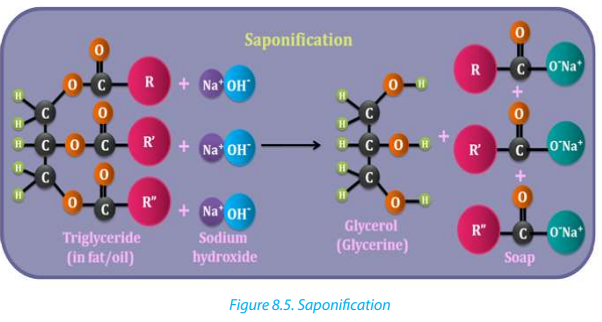
The reaction of saponification is exothermic because there is liberation of heat and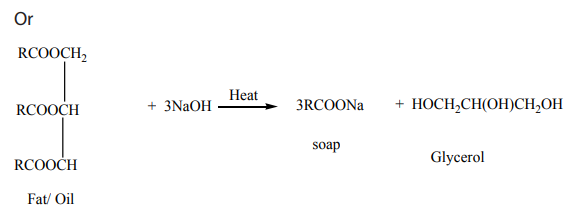
the soap formed remains in suspension form in the mixture. Soap is precipitated asa solid from the suspension by adding common salt to the suspension.
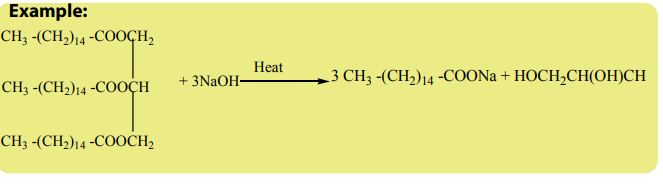
Note: Sodium soaps are “hard” soaps whereas potassium soaps are soft.
DETERGENTS
Detergents are organic liquid or water-soluble solid cleaning substances that, unlike
soap, are not prepared from fats and oils.
The chemical composition of detergents is different from that of soaps but they
have the same cleaning mechanism and are not adversely affected by hard minerals
in the water and this makes them more effective than soaps. However, they are less
environmental friendly because of a reduced biodegradability.
Detergents may be used for household cleaning, laundry or for body and hand
washing. They exist in the powder or liquid form.
How do soaps and detergents work?
When a soap or detergent is added to water, a polar solvent, the molecules form
clusters, known as micelles(Figure 8.6), in which the polar ends of the molecules areon the outside of the cluster and the non-polar ends are in the middle.
The carboxylate end of the soap molecule is attracted to water. It is called the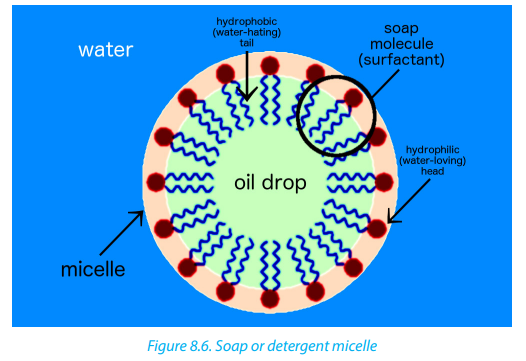
hydrophilic (water-loving) end. The hydrocarbon chain is attracted to oil and grease
and repelled by water. It is known as the hydrophobic (water-hating) end. When
washing, the hydrophobic part of the soap molecule (Figure 8.7) dissolves oil or
grease the main source of dirt and it gets washed away by water as it is insoluble init.
The other major soap-making process is the neutralization of pure fatty acids withan alkali.
The cleaning property of both soaps and detergents results from their capacity
to emulsify water-insoluble materials (dirt, oil, grease, etc.) and hold them in
suspension in water. This ability originates from the molecular structure of soaps
and detergents. When a soap or detergent adds on to water that contains oil or
other water-insoluble materials, the soap or detergent molecules surround the oil
droplets. The oil or grease is “dissolved” in the alkyl groups of the soap molecules
while the ionic end allows the micelle to dissolve in water. As a result, the oil droplets
are dispersed throughout the water (this is referred to as emulsification) and can berinsed away.

Difference Between Soap and Detergent

Checking Up 8.3.
1. Propyl tristearate reacts with sodium hydroxide to form soap.
a. Write a balanced equation for the reaction which takes place.
b. Calculate the mass of sodium hydroxide needed to react exactly with
4kg of this oil and the mass of the produced soap.
2. Describe the chemical difference of solid and liquid soaps.
3. Distinguish soaps from detergents.
4. Why are detergents more effective than soaps?
5. Describe briefly in your own words how soaps and detergents work.6. Discuss the importance of soaps and detergents in our everyday life.
8.4. Structure and nomenclature of acid anhydrides
One of the most used pain killers is aspirin. This is a medical drug which can be
prepared using salicylic acid and ethanoic acid. However, ethanoic acid is not
used. Instead, one of its derivatives is used. Search from internet or the school
library and answer the questions below:
1. Propose a derivative of acetic acid is used in this preparation?
2. Explain why is it used in preference to acetic acid?
3. Write down its molecular formula and structure.4. Suggest how it is produced from acetic acid.
8.4.1. Structure of acid anhydrides
The acid anhydrides are derivatives of carboxylic acids.
The general structure of acid anhydrides is RCOOOCR, or
general structure of acid anhydrides is RCOOOCR, or
When the two R groups are identical, the acid anhydride is symmetric and when thetwo R groups are different, the acid anhydride is asymmetric. The general molecular
formula of acid anhydride is
The functional group of acid anhydrides consists of two acyl groups held togetherby an oxygen atom.
When the two R groups are identical, the acid anhydride is symmetric and when the
two R groups are different, the acid anhydride is asymmetric. The general molecularformula of acid anhydride is
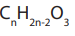
8.4.2. Nomenclature of acid anhydrides
The nomenclature of acid anhydride is based on whether they are symmetrical or
unsymmetrical. Symmetrical acid anhydrides are named as parent acid followed bythe term anhydride

Unsymmetrical acid anhydrides are named by writing alphabetically the names of
parent acids followed by the term anhydride.

Checking up 8.4.
1. Write the molecular formula of an acid anhydride which has 6 carbon
atoms
2. Draw the structure of one straight and one branched isomers of the
molecular formula in (1) above.3. Name the isomers from (2).
8.5. Preparation, chemical properties and uses of acid anhydrides
Activity 8.5.
1. Two carboxylic acids can react to form an acid anhydride and a water
molecule. However, this method is not suitable when preparing mixed
(unsymmetrical) acid anhydrides.
2. Suggest a reason why this method is not suitable.
3. Using your knowledge in organic chemistry so far, suggest a method
which may be suitable to prepare ethanoic propanoic anhydride. Write
the equation for the reaction. (Hint: you may refer to the preparation of
ethers).
4. Prepare ethanoic anhydride using ethanoic acid and phosphorous
pentoxide.
5. Aspirin is synthesized using ethanoic anhydride and salicylic acid. Suggestan equation for the reaction that occurs.
8.5.1. Preparation
Anhydride means “without water”. Two carboxylic acids can react, eliminating awater molecule to yield an acid anhydride.
The commonly used dehydrating agent is phosphorous pentoxide,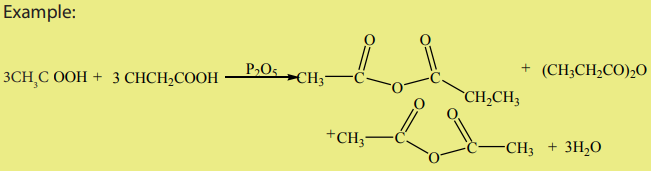
 If two
If two
different acids are used, a mixed anhydride is produced. The method is less efficient
however, as one obtains the two symmetrical anhydrides in addition to the desired
mixed anhydride.
A better method of making mixed anhydrides is to react an acid halide with a saltof a carboxylic acid. This method can be used to make symmetrical anhydrides too.
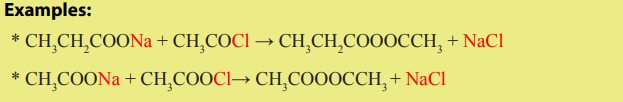
8.5.2. Chemical properties of acid anhydrides and their uses
The term “chemical properties” indicates the reactivity between two or more
compounds. In the case of acid anhydrides, their reactivity involves the electron deficient carbonyl-carbon which is attacked by nucleophiles. This reaction occurs slowly.
There are mainly four types of reactivity of acid anhydrides such as hydrolysis, reaction
with alcohols, reaction with ammonia and amines and the reduction reaction.
1. Hydrolysis
This reaction of acid anhydride in water leads to the formation of parent carboxylic
acids which were used to prepare the anhydride. The reaction is carried out in acidicmedium under reflux.

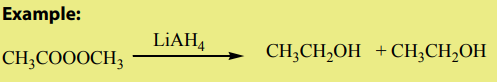
2. Reaction with alcohols
Anhydrides react readily with primary, secondary, tertiary alcohols to form estersand carboxylic acid.

Aspirin synthesis is an application of this reaction
This reaction is very important in pharmaceutical industries and it indicates the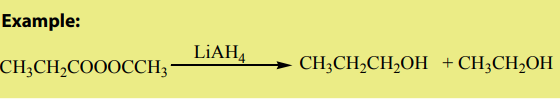
main use of acid anhydrides as it is the basis of aspirin manufacture as shown below.
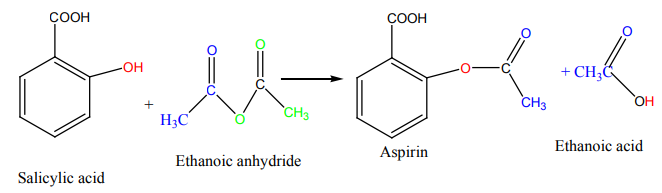
3. Reaction with ammonia and amines
Anhydrides react with ammonia, primary and secondary amines to produce
amides.
The reaction with amide:

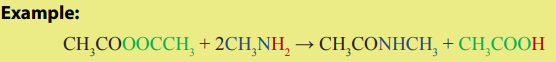
Reaction with ammonia: (RCO)2 O + NH3 → RCONH2 + RCOOH
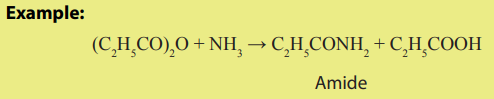
4. Reduction of acid anhydrides
Anhydrides are reduced by Lithium tetrahydridoaluminate,
to yield two moles of primary alcohols.
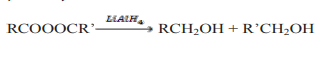
Where R and R can be hydrogen atoms (for primary amides), alkyl groups (fortertiary amides). For secondary amides only one R is a hydrogen atom. Their general
molecular formula is Cn
H2n+1ON. Examples of some amides are given in the Table 8.2.
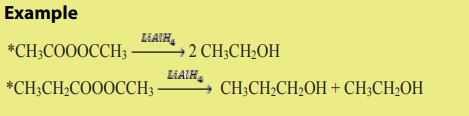
8.5.3. Uses of acid anhydrides
1. Ethanoic anhydride is used in the synthesis of acetate esters.
2. Examples: aspirin, cellulose acetate,…
3. Maleic anhydride is used in the synthesis of various resins when
copolymerized with styrene.4. They are used to synthesize polyesters and polyamides.
Checking up 8.5
1. Write the equations that can be used to synthesize the following acid
anhydrides from ethanol.
a. Ethanoic anhydride
b. Propanoic anhydride
c. Ethanoic propanoic anhydride
2. Students of senior five MCB were asked to prepare butanoic propanoic
anhydride and group A used a method similar to Williamson’s method of
synthesizing ethers whereas group B decided to use a dehydrating agent.
Which group chose a better method? Explain your reasoning3. Complete the equations below
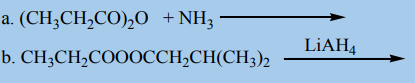
4. Propose the products from the reaction below:
5.Draw the structures of products formed when propanoic anhydride reacts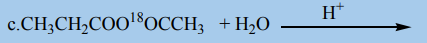
with 2-methylpropan-2-ol
6. State the necessary chemicals and conditions to prepare aspirin.
7.Chemists prefer using acid anhydrides than acyl chlorides when preparingesters. Discuss this statement.
8.6. Structure and nomenclature of amides
Activity 8.6.
In the previous unit, it has been mentioned that carboxylic acids react with
ammonia and amines to produce new organic compounds.
1. Draw and name their functional group.
2. Draw their general structure and determine their general molecular
formula.
3. What natural and artificial polymers contain the same functional group?
4. Suggest how their boiling points would be relative to those of esters.Provide an explanation for your suggestion.
8.6.1. Structure of amides
Amides are acid derivatives in which the –OH group is replaced by or
or
. The functional group comprises nitrogen atom which is attached to the
carbonyl carbon atom. The carbonyl group linked to nitrogen atom is called anamide linkage. The general structure of amides is:
Where R and R can be hydrogen atoms (for primary amides), alkyl groups (for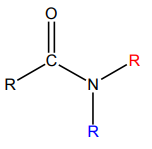
tertiary amides). For secondary amides only one R is a hydrogen atom. Their general
molecular formula is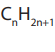 ON. Examples of some amides are given in the Table 8.2.
ON. Examples of some amides are given in the Table 8.2.Table 8.2. Examples of some amides

8.6.2. Nomenclature of amides
The nomenclature of amides is derived from the name of corresponding acid. The
–oic acid suffix or –ic acid is replaced by –amide.
As for other organic compounds, the first step is to consider the number of carbon
atoms forming the chain.
The alkyl group bonded to nitrogen atom is indicated by a capital N preceding thealkyl name.
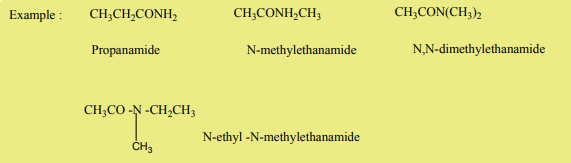
8.6.3. Physical properties and uses of amides
Physical properties of amides
Except formamide, all the amides are crystalline solids at room temperature. Amides
have higher melting and boiling points than corresponding esters due to hydrogenbonding as shown below.
The melting and boiling points increase as molecular mass increases. Lower members
are soluble in water but this solubility decreases as the molecular mass increases. All
the amides are soluble in organic solvents. The Table 8.3 shows the comparison ofmelting and boiling points of some amides.
Table 8.3. Some physical properties of lower amides
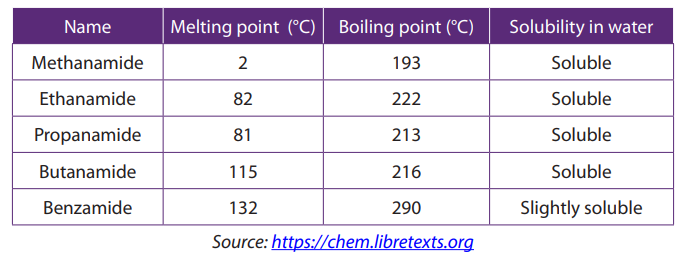
8.6.4. Uses of Amides
Amides are used in the production of many useful chemicals and materials such asfertilizers (urea), nylon textiles and skin care substances.
Urea manufacture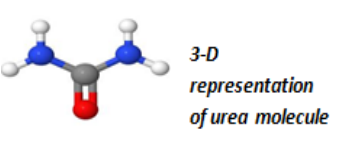
Urea can be prepared in three ways:a. Reaction of phosgene and ammonia

The diagram below shows the representation of Urea.
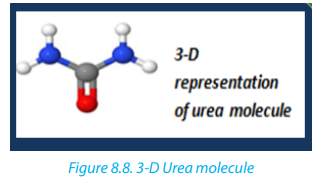
b. From calcium cyanamide,
Calcium carbide reacts with nitrogen to produce calcium cyanamide and carbon.
 produced
produced  is then treated with a mixture of water
is then treated with a mixture of water and carbon dioxide to produce urea.

c. Reaction of carbon dioxide and ammonia
Urea is also naturally present in animal urines.
It is widely used in agriculture as a source of nitrogen, chemical fertilizer. It also finds
use in animal feeding and in resins manufacture.
Nylon manufacture
Nylon-6,6 is a synthetic textile produced when hexane-1,6-dioic acid(adipic acid)
reacts with hexane-1,6-diamine. Nylon is a polyamide. Materials and clothes aremade from nylo-6,6.
Medical use of urea
Urea containing creams are used in skin treatment to promote its rehydration. It softens theskin.
Checking up 8.6.
1. Write the molecular formula of amides with 4 carbon atoms
2. Draw all possible structural formulae of primary, secondary and tertiary
amides with molecular formula in (1) above and name them
3. Compare the solubilities of butanamide and N,N-dimethylethanamide
in water
4. The solubility of amides decreases with the increase in molecular mass.
Suggest a reason for this observation.
5. Which one between ethanol and ethanamide do you expect to have a
higher boiling point? Explain your answer.
6. Discuss the benefits and dangers of using animal urine as a source ofnitrogen for plants.
8.7. Preparation and chemical properties of amides
Activity 8.7.
1. Draw the structure of propanamide.
2. Suggest how this compound can be prepared from propanoic acid.
Include an equation in your answer and state working conditions.
3. Draw the structure of ethanoyl chloride and write an equation for its
reaction with
4. Suggest other possible reactions that can be used to prepare amides in
general5. What reagents and conditions which can be used to reduce amides?
8.7.1. Preparation of amides
Amides can be prepared from all of the other acid derivatives when they react with
ammonia and primary or secondary amines. Their production of amides involvesthe following reactions.
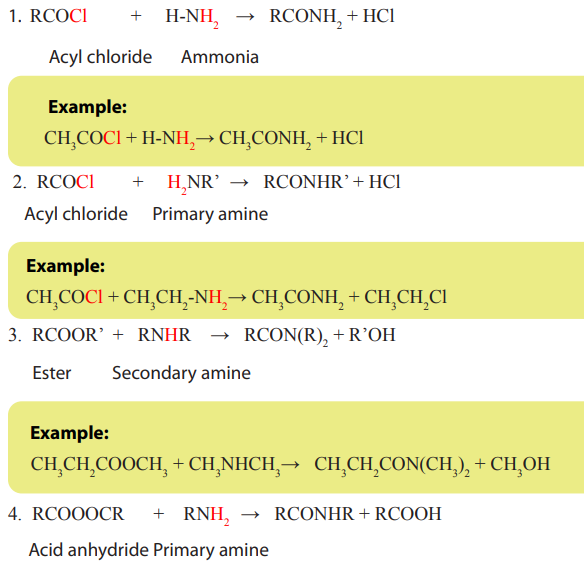

8.7.2. Chemical properties of amides
The reactivity of amides involves different types of reaction to form various organic
compounds.
1. Reduction reaction
Amides are reduced with sodium and ethyl alcohol or with lithium aluminium
hydride
 to yield primary amines.
to yield primary amines.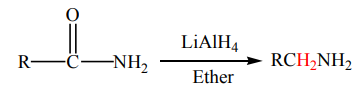

2. Hydrolysis
Amides react with water in acidic medium (dilute) at high temperatures to formacids.


3. Hoffman degradation
Amides react with a mixture of sodium hydroxide and bromine or sodium
hypobromite to produce amines. The reaction is called degradation as the carbonchain is reduced by one carbon.


This equation can be simplified as follows:
Note: Hoffman degradation reaction is used to test the presence of the amide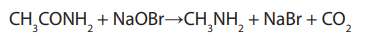
functional group. When an amide is treated with sodium hypobromite, acolorless gas which turns milky lime water is evolved,
4. Reaction with nitrous acid
Amides react with nitrous acid to produce an acid, water and nitrogen gas.
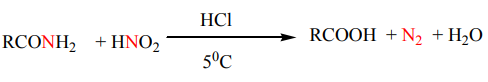
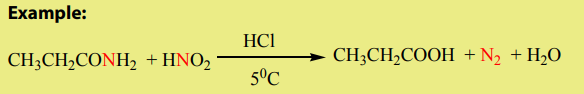
5. Dehydration reaction
Dehydrating reagents, like thionyl chloride remove one molecule of water
remove one molecule of water
from amides to give nitriles. Phosphorous pentoxide can also be used. The reactionis done under reflux.

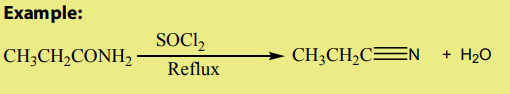
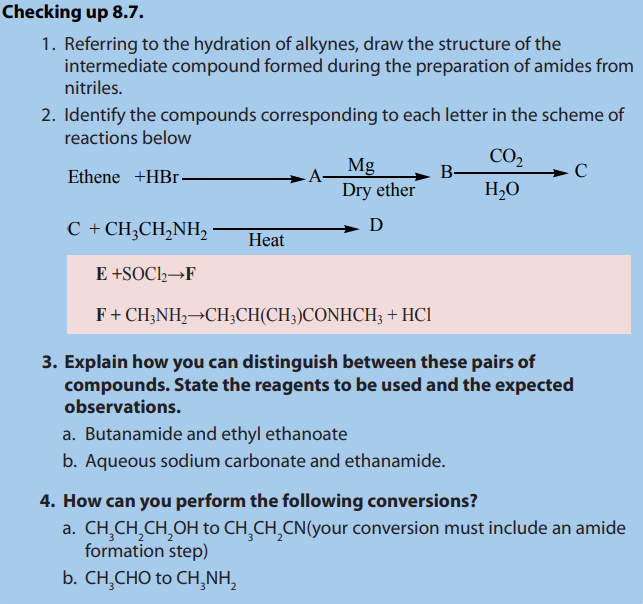
8.8. Structure and nomenclature of nitriles
Activity 8. 8.
In the previous lesson of this unit you learnt different reactions of amides. Fromyour knowledge and understanding, answer the questions that follow:
1. Draw the structure of compound A:
2. To what homologous series does product A belong?
3. Write the molecular formula of A.
4. Suggest the general structure and the general molecular formula for all
compounds belonging to the same homologous series as A.
5. At room temperature, these compounds are liquids or solids depending
on the molecular mass and yet they lack hydrogen bonding. Suggest abrief explanation for this specialty.
8.8.1. Structure of nitriles
Nitriles are organic compounds with the general structure is its
is its
functional group. The nitrile compounds include a nitrogen atom attached to a
carbon atom by a triple covalent bond. Their general molecular formula is
Unlike other acid derivatives they do not contain an acyl group.
8.8.2. Nomenclature of nitriles
The nitriles are named using the name of the alkane parent followed by the term
–nitrile. The carbon attached to the nitrogen atom is given the location positionnumber 1.
Structure and name of some nitriles are shown in the Table 8.4.

8.8.3. Physical properties and uses of nitriles
Physical properties
The physical properties of nitriles are summarized below.
1. The nitrile compounds are present as colorless solids and liquids having a
characteristic odor.
2. Nitriles have boiling points ranging between 82 and 118 °C. The high
boiling points are due to strong dipole-dipole moments caused by the
polarity of the C N bond.
3. Nitriles compounds exhibit high polar and electronegativity
4. Lower nitriles are highly soluble in water but this solubility decreaseswith the increase in molecular mass as the non-polar part becomes lager.
Uses of nitrile compounds
Nitriles find many uses:
• Nitriles are used in the manufacture of nitrile gloves, seals, and pipes or tubes
as they exhibit resistance to chemicals.
• They are used as an antidiabetic drug which is used in the treatment of breast
cancers.
• This compound is found in many plant and animal sources.
• They are utilized in the applications of oil resistant substances and also for
low-temperature uses
They are also employed in automotive systems, hydraulic tubes and also in aircraftsystems.
Checking up 8. 8
1. Draw the structure of each of the compound below:
a. Butanenitrile
b. 3-methylpentanenitrile2. Name these compounds:

3. Draw all possible isomers of molecular formula
 and name them.
and name them.8.9. Preparation and chemical properties of nitriles
Activity 8. 9
One method of preparing nitriles is to dehydrate an amide.
1. Use your knowledge about chemistry of alkyl halides and suggest
another preparation method.
2. Name the reaction mechanism involved in that method
3. Write an equation of the preparation of propanenitrile using the methodyou have suggested.
8.9.1. Preparation of nitriles
Nitriles are prepared by dehydration of amides under reflux in the presence
of phosphorous (V) oxide, or sulphur dichloride oxide,
or sulphur dichloride oxide, 
and there is elimination of water molecule.
1.Dehydration of amides

2.Nucleophilic substitution of halogenoalkanes
The halogenoalkane is heated under reflux with a solution of sodium or potassium
cyanide in ethanol. The halogen is replaced by a -CN group and a nitrile isproduced.
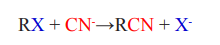
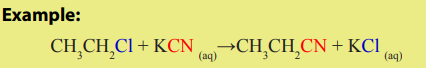
8.9.2. Chemical properties of Nitriles
Nitrile compounds undergo various reactions. Nitriles are hydrolyzed in the
presence of an acid or a base to form carboxamides and carboxylic acids. This is
the reason why they are considered to be acid derivatives while they have no acylgroup.
1. Hydrolysis

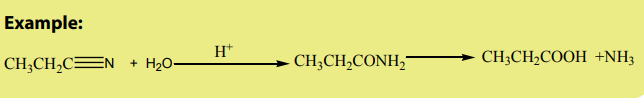
2. Reduction reaction.
Nitriles can be reduced by to produce primary amines in the presence of
to produce primary amines in the presence of catalysts such as


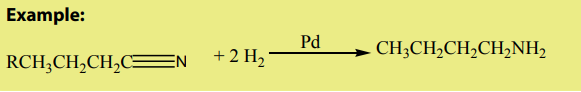
Checking up 8. 9.
1. An aldehyde of molecular formula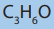 reacts with hydrogen cyanide
reacts with hydrogen cyanide
in strong basic medium to give compound A. compound A undergoes a
reduction to give compound B.
a. Suggest a reducing agent which can be used to reduce A.
b. Draw the structure of the product formed when compound A is treated with hot acidified water
2. What is meant by the term hydrolysis?
3. Nitriles are considered to be acid derivatives even though they do not
have the acyl group. Search from the internet or library a reason for thisconsideration.
8.10. End Unit assessment
Part I: Objective questions
1. The esters are ……. acyl chlorides
d. more reactive than
e. equal in reactivity
f. less reactive than
2. Secondary amines react with acid chloride to give
a. amines
b. carboxylic acids
c. amides
d. imines
3. A mixture of acetic acid and propanoic acid undergo dehydration to give
a. acetic anhydride
b. propanoic anhydride
c. acetic and propanoic anhydrides
d. acetic, propanoic and acetic propanoic anhydrides
4. Ethanoyl bromide reacts with sodium propanoate to give
c. ethanoic anhydride
d. propanoic anhydride
e. ethanoic propanoic anhydride
f. all of the above
5. Esters are made from the reaction between
a. carboxylic acid molecules
b. alcohol molecules
c. alcohol and carboxylic acid molecules
d. acid anhydride and water molecules
6. Ethyl acetate is hydrolyzed by water to give a/an
a. lactone
b. ester
c. acid anhydride
d. carboxylic acid and an alcohol
7. The reaction between ethyl ethanoate and dimethylamine gives an
a. amide
b. imide
c. acid anhydride
8.
reduces Ethanamide to give a/an
a. carboxylic acid
b. amide
c. alcohol
d. amine
9. Nitriles can be hydrolyzed with water to give
a. alcohols
b. aldehydes
c. acidsd. acids and amides
10. Reduction of nitriles gives
e. amide
f. amine
g. imine
h. carboxylic acids
Part II: Structured questions
1. Use equations to show how you could prepare the following compounds,
using the
organic compounds cited as the only organic substances and any inorganicsubstance you need:
5. Draw the structural formula of:
a. 2-chloropropanamide
b. Methylpentanoate
c. Butanoic anhydride
d. Propanoyl chloride
e. N-ethyl-N-propylbutanamide
6. Give the organic products of the following reactions:
a. Propanoic acid and ammonia.
b. Ethanoyl chloride plus methanol.
c. Butanoic anhydride plus water.
d. Propanamide plus sodium hypobromite
e. Ethanol plus propanoyl chloride
7. Give reagents, essential conditions and equations for the conversion of
ethanoic acid into:
a. Ethanoic anhydride
b. Ethanamide
c. Ethyl ethanoate
8. Ethanoic anhydride is a liquid at room temperature but Ethanamide is a
solid. Comment briefly on this.9. Discuss the uses of esters.
10. a. Write an equation for the formation of ethyl ethaonate from ethanoyl
chloride and ethanol. Name and outline the mechanism for the reaction
taking place.
Explain why dilute sodium hydroxide will cause holes in clothing made from
polymers such as terylene while polythene containers can store caustic soda.11. Ethyl oleate is an ester with the molecular structure below:
It is possible the body could synthesize this compound from the ethanol present
in alcoholic drinks and the natural fatty acid, oleic acid.
a. Write the structural formula of oleic acid
b. Construct a balanced equation for the production of ethyl oleate from
ethanol and oleic acid.c. Suggest how oleic acid can be obtained from the triglyceride below
12.This question is about the reactions of carboxylic acids and their derivatives.
a. A carboxylic derivative X was found to contain C, H, N and O. analysis
gave the following percentage composition by mass: 49.4%, 9.6% and
19.1% for carbon, hydrogen and nitrogen respectively. Compound X had
a relative molecular of 73.
i. Calculate the empirical and molecular formulae of X.
ii. Suggest three possible structures of X.
b. Acyl chlorides such as ethanoyl chloride undergo several reactions due
to their high reactivity. What could be produced when ethanoyl chloride
reacts with:
c. A and B are two isomeric amides which can be hydrolyzed in acidic medium.
i. Water
ii. Propan-2-ol
iii. Ammoniaiv. Sodium acetate
i. Draw the structures of the products formed from hydrolyzing A and B.
ii. What is the structure of the compound produced when A reacts with
sodium hypobromite?iii. Write an equation for the reaction of B with ethanoyl chloride.
UNIT 9: AMINES AND AMINO ACIDS
Key unit competency:
The learner should be able to relate the chemical nature of the amines andaminoacids to their properties, uses and reactivity.
Learning objectives
At the end of this unit, the students will be able to:
• Explain the zwitterion forms in the solution of different pH.
• Explain the isoelectric point in amino acids.
• Describe the physical properties and uses of amines.
• Describe the preparation methods of the amines.
• Describe the reactions of amino acids and amines with other substances.
• Classify amines as primary, secondary and tertiary amines.
• Compare and contrast the physical properties of the amino acids to those of
carboxylic acids and amines.• Test the presence of amines and amino acids in the solution.
Introductory activity
Read the text below, observe the accompanying images and answer the
questions.
The cell is the basic structural, functional, and biological unit of all known living
organisms. A cell is the smallest unit of life. All cells are made up of Inorganic
compounds and Organic compounds. Inorganic compounds are compounds
that do not contain carbon atoms except carbonates ,carbides, carbon oxides
,carbonic acid while organic compounds are compounds that mainly contain
carbon (C) and hydrogen (H) atoms, and eventually other elements such as
oxygen (O) and nitrogen (N) in biological macromolecules that include thecarbohydrates, lipids, proteins and nucleic acids.
Proteins are macromolecules consisting of one or more long chains of amino
acid residues. Proteins differ from one another primarily in their sequence of
amino acids which is dictated by the nucleotide sequence of their genes,
and which usually results in protein folding into a specific three-dimensional
structure.
That determines its activity. Nucleic acids [Deoxyribonucleic acid (DNA) and
ribonucleic acid (RNA)] alongside proteins, lipids and complex carbohydrates
(polysaccharides), are one of the four major types of macromolecules that are
essential for all known forms of life. They are a thread-like chain of nucleotides
carrying the genetic instructions used in the growth, development, functioning
and reproduction of all known living organisms and many viruses.
The image below shows the partial molecular structure of DNA (A) and ofproteins (B, C).
1. Identify the common point between DNA and proteins. Explain your
answer.
2. The terminology “amino acid” is found in the text and image above.
According to you, what is it, and what is its role in living organisms?
3. If protein molecules are made essentially of Carbon, Hydrogen, Oxygen,
Nitrogen and amino acid side chains (figure C),
a. What kind of bonds do you expect to see in those structures?b. What kind of reactions do you expect in those molecules?
9.1. Nomenclature and classification of amines
Activity 9.1
Pentan-2-ol, butan-1-ol and 2-methylpropan-2-ol are alcohols.
1. For each one:
a. give its molecular formula
b. give its structural formula
c. give its displayed formula
d. give its skeletal formula
e. State whether it is a primary, secondary or tertiary alcohol.
2. Give the general formula that is used to represent alcohols.
3. Two of the alcohols in this question are isomers of each other. Identifywhich two and identify the type of isomerism they show.
4. Name the alcohol whose structural formula is

Amines are one of organic compounds containing nitrogen. They are one of the
most important classes of organic compounds which are obtained by replacing
one or more hydrogen atoms by an alkyl or aryl group in a molecule of ammonia They are present in vitamins, proteins, hormones, etc. They are extensively
They are present in vitamins, proteins, hormones, etc. They are extensively used in the manufacturing of many drugs and detergents.
9.1.1 Classification of amines
As per VSEPR theory, nitrogen present in amines is sp3
hybridized and due to the
presence of lone pair, it is pyramidal in shape instead of tetrahedral shape which is a
general structure for most hybridized molecules. Each of the three
hybridized molecules. Each of the three 
hybridized orbitals of nitrogen overlap with orbitals of hydrogen or carbon depending
upon the configuration of amines. Due to the presence of lone pair, the C-N-H angle in amines
is less than 109 degrees which is characteristic angle of tetrahedral geometry. Theangle in amines is near about 108 degrees.
The functional group of amines is Depending upon the number of
Depending upon the number of
hydrogen atoms that are replaced by an alkyl or aryl group in ammonia, amines areclassified as primary, secondary and tertiary (Table 9.1).
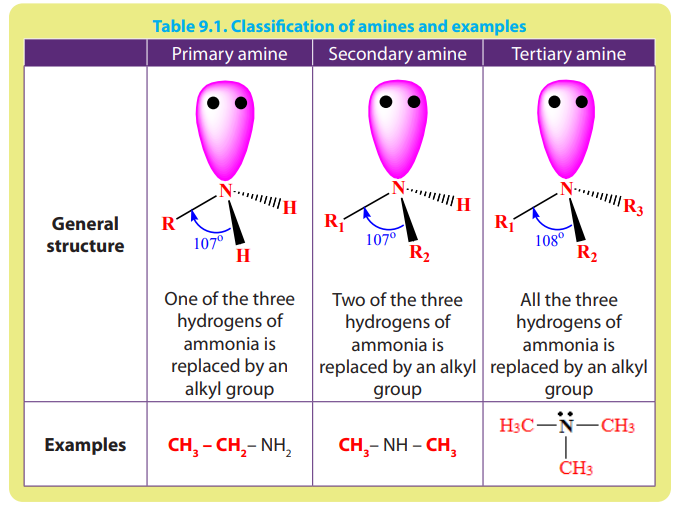
9.1.2 Nomenclature of amines
In organic chemistry, the names of the compounds are given according to the
guidelines provided by IUPAC. In this regards, amines are named by ending with –
amine. The IUPAC system names amine functions as substituents on the largest alkylgroup.
If the amine is secondary and has two alkyl groups attached to the nitrogen, then
each chain is named and the smaller alkyl group is preceded by an –N which playsthe same role as a number in positioning a side alkyl chain.
N-methylpropylamine is the common name and N-methyl-1-aminopropane
is the IUPAC name.
If in the common naming the lengths of the chain are the same, an –N is not usedas shown in the following example:
 (Diethylamine is the common name which does not
(Diethylamine is the common name which does not
contain N because the chains have the same length and N-ethylethanamine isthe IUPAC.
case of a tertiary amine, the use of N is applied at each alkyl side group.
The following are examples of primary, secondary and tertiary amines (Table 9.2)
with their corresponding names using IUPAC of common name.
Aliphatic and Aromatic and heterocyclic amines are named after the groups
surrounding the nitrogen + amine.
In case of heterocyclic amines, there is a systematic nomenclature of these
compounds as indicated in the following examples.
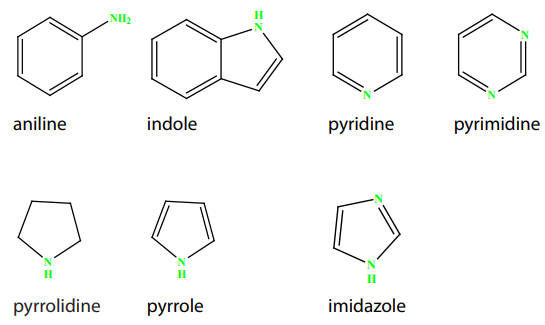
Checking up 9.1
1. Classify the following as primary, secondary and tertiary amines.
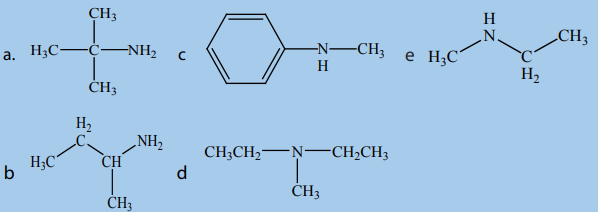
2. For each compound below, provide the respective IUPAC name.
3. Write IUPAC names of the following compounds and classify them into
primary, secondary and tertiary amines.
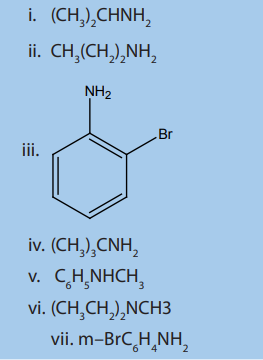
9.2 Physical properties, natural occurrences and uses of amines.
Activity 9.2
Calculate the molecular mass of the given products and justify the differencebetween boiling points of amines, alkanes and alcohols

9.2.1 Physical properties of amines
Primary and secondary amines can form a hydrogen bond to each other (as shown
in the Figure 9.1 below where two molecules of are bonded together).
are bonded together).
Because Nitrogen is less electronegative than oxygen, the N—H bond is not quite
as polar as the O—H bond reason why hydrogen bonds in amines are not strong
than these alcohol molecules. Therefore primary and secondary amines have lowerboiling points than alcohols of similar molecular weight.
Tertiary amines do not bond to each other by hydrogen bond and they have boiling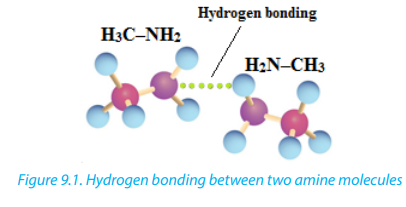
points similar to those of hydrocarbons of the same molecular weight. However,
primary, secondary and tertiary amines form hydrogen bond with water and amineswith low-molecular weight are generally soluble in water.
Generally the boiling point of amines increases as the molecular weight increase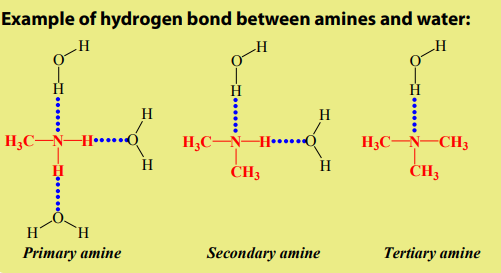
and they boil at higher temperatures than alkanes but at lower temperatures than
alcohols of comparable molar mass.
The amines are soluble in organic solvent and the solubility decreases as the
molecular weight increases. The Table 9.3 summarizes some physical properties ofsome amines.
Table 9.3: Physical pproperties of some amines compared to someoxygen containing compounds.
Amines tend to be gases at low molecular weight (e.g. up to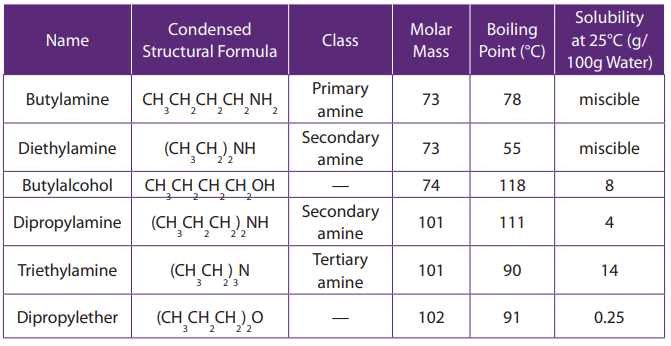
 trimethylamine)
trimethylamine)
and the heavier ones are liquids at room temperature. In fact, methyl, dimethyl,
trimethyl, and ethyl -amines are gases under standard conditions. Most common
alkyl amines are liquids, and high molecular weight amines are, quite naturally, solids
at standard temperatures. Additionally, gaseous amines possess a characteristic
ammonia smell, while liquid amines have a distinctive “fishy” smell: higher molecular
weight amines often smell like rotting fish, and are often found in decaying
animal tissues. Cadaverine and putrescine
and putrescine  are some
are some
of the examples. The lower molecular- weight amines with up to about five carbon
atoms are soluble in water. The higher-molecular-weight amines that are insolublein water will dissolve in acid to form ionic amine salts.
9.2.2 Natural occurrence of amines and their usage
Natural amines occur in proteins, vitamins, hormones, etc. and they are
also prepared synthetically to make polymers, drugs and dyes.
Amines can be used as dyes (colorants) or as drugs: Primary aromatic amines are
used as a starting material for the manufacture of azo dyes. They react with nitrous
(II) acid to form diazonium salt which can undergo a coupling reaction in order to
form an azo compound. As azo compounds are highly coloured, they are widelyused in dyeing industries. Examples include Methyl orange and Direct brown 138.
In medicine, amines can be used as drugs.
• Chlorpheniramine is an antihistamine that helps to relief allergic disorders
due to cold, hay fever, itchy skin, insect bites and stings.
• Diphenhydramine is the common antihistamine.
• Chlorpromazine is a tranquillizer that anaesthetizes without inducing sleep.
It is used to relieve anxiety, excitement, restlessness or even mental disorder.
• Acetaminophen is also known as paracetamol or p-acetaminophenol, it is
an analgesic that relieves pains such as headaches. It is believed to be less
corrosive to the stomach and is an alternative to aspirin.
Amines are widely encountered in biological and pharmacological studies. Some
important examples are the 2-phenylethylamines, some vitamins, antihistamines,
tranquilizers, and neurotransmitters (noradrenaline, dopamine and serotonin)which act at neuromuscular synapses.
Checking up 9.2
1. Which compound of each pair has the higher boiling point? Explain.a. butylamine or pentane
2. Between the two compounds
 explain which
explain which
is more soluble in water.
9.3 Preparation of amines.
Activity 9.3
Ammonia molecules react with water molecules. Write the detailed
molecules react with water molecules. Write the detailed chemical equation of that reaction.
The amines can be prepared based on the following reactions:
9.3.1 Alkylation of ammonia
Primary amines can be synthesized by alkylation of ammonia. The reaction involves
nucleophilic substitution of an alkyl halide when ammonia is used as nucleophilicagent. The reaction is carried out in a sealed tube at 100 °C or 373 K.

9.3.2. Gabriel phthalimide synthesis
This procedure is used for the preparation of primary amines. Phthalimide on
treatment with ethanolic potassium hydroxide forms potassium salt of phthalimide
which on heating with alkyl halide followed by alkaline hydrolysis produces the
corresponding primary amine. However, primary aromatic amines cannot be
prepared by Gabriel phthalimide synthesis because aryl halides do not undergonucleophilic substitution with the anion formed by phthalimide.

9.3.3. Hoffmann bromamide degradation reaction
Hoffmann developed a method for the preparation of primary amines by treating an
amide with bromine in an aqueous or ethanolic solution of sodium hydroxide. This
is a degradation reaction with migration of an alkyl or aryl group taking place from
carbonyl carbon of the amide to the nitrogen atom.
The reaction is valid for the preparation of primary amines only, and it yieldsuncontaminated compound with other amines.
9.3.4 Reduction of amides
Similarly to reduction of amides, lithium aluminium hydride reduces
reduces amides to amines.
9.3.5 Reduction of nitriles
Nitriles are reduced to amines using hydrogen in the presence of a nickel catalyst,
although acidic or alkaline conditions should not be used to avoid the possible
hydrolysis of the -CN group. is more commonly employed for the reduction of
is more commonly employed for the reduction of nitriles on the laboratory scale.

9.3.6 Reduction of nitro compounds
Nitro compounds are reduced to amines by passing hydrogen gas in the presence
of finely divided nickel, palladium or platinum and also by reduction with metals
in acidic medium. Nitroalkanes can also be similarly reduced to the correspondingalkanamines.
Reduction with iron scrap and hydrochloric acid is preferred because
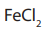
formed gets hydrolysed to release hydrochloric acid during the reaction.
Thus, only a small amount of hydrochloric acid is required to initiate the reaction.Checking up 9.3
1. Give two reagents that could be used to synthesize the followingreaction.
2.Discuss the conditions for using each of the reagents of your choice.
3.Write chemical equations for the following reactions:
a. Reaction of ethanolic
b. Ammonolysis of benzoyl chloride and reaction of amine so formed withtwo moles of
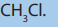
9.4. Chemical properties of amines
Activity 9.4
Experiment: In a solution of ethylamine at room temperature (A), add dilute
hydrochloric acid (B). After a while (C), add excess of sodium hydroxide in the
solution (D) to obtain the solution E.
Explain:
1. What evidence is there for a chemical reaction between ethylamine and
hydrochloric acid?
2. Why does the smell of ethylamine disappear when hydrochloric acid is added?
3. Why does the smell reappear when sodium hydroxide is added?Difference in electronegativity between nitrogen and hydrogen atoms and the
presence of unshared pair of electrons over the nitrogen atom makes amines
reactive. The number of hydrogen atoms attached to nitrogen atom also is involved
in the reaction of amines; that is why the reactivity of amines differ in many reactions.
Amines behave as nucleophiles due to the presence of unshared electron pair.
The chemical properties of amines are summarized in the reactions below.
9.4.1 Reactions of amines diluted with acids
Amines, like ammonia, are bases. Being basic in nature, they react with acids toform salts.
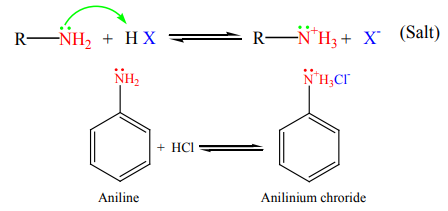
Amine salts on treatment with a base like NaOH, regenerate the parent amine.

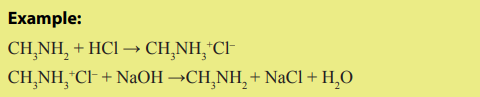
9.4.2 Reactions of amines (alkylation, acylation, and sulfonation)
Acyl chlorides and acid anhydrides react with primary and secondary amines to
form amides. Tertiary amines cannot be acylated due to the absence of a replaceablehydrogen atom.

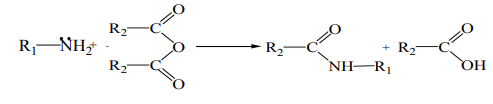
9.4.3. Reaction with carboxylic acid
Because amines are basic, they neutralize carboxylic acids to form the corresponding
ammonium carboxylate salts. Upon heating at 200°C, the primary and secondaryamine salts dehydrate to form the corresponding amides.
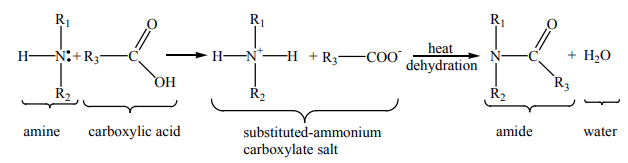
9.4.4. Reaction with nitrous acid
Nitrous acid,
 is unstable. It is produced indirectly using a mixture of
is unstable. It is produced indirectly using a mixture of 
and a strong acid such as
 in diluted solution. Primary aliphatic amines react
in diluted solution. Primary aliphatic amines react with nitrous acid to produce a very unstable diazonium salts which spontaneously
decomposes by losing
 to form a carbenium ion. Further, the carbonium ion is
to form a carbenium ion. Further, the carbonium ion is used to produce a mixture of alkenes, alkanols or alkyl halides, with alkanols as
major product said above.

Primary aromatic amines, such as aniline (phenylamine) forms a more stable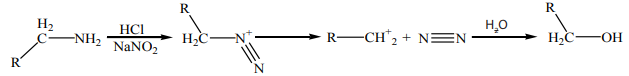
diazonium ion at Above 5°C, it will decompose to give phenol and
Above 5°C, it will decompose to give phenol and 
Diazonium salts can be isolated in the crystalline form but are usually used in solutionand immediately after preparation, due to its rapid decomposition.

9.4.5. Reactions with ketones and aldehydes
Primary amines react with carbonyl compounds to form imines. Specifically,
aldehydes become aldimines, and ketones become ketimines. In the case offormaldehyde (R’ = H), the imine products are typically cyclic trimers.
Secondary amines react with ketones and aldehydes to form enamines. An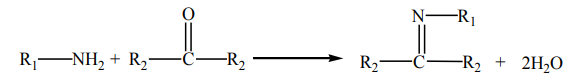
enamine contains a C=C double bond, where the second C is singly bonded to N aspart of an amine ligand.
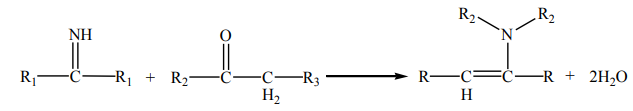
9.4.6. Neutralization reactions
Tertiary amines react with strong acids such as hydroiodic acid (HI), hydrobromic
react with strong acids such as hydroiodic acid (HI), hydrobromic
acid (HBr) and hydrochloric acid (HCl) to give ammonium salts
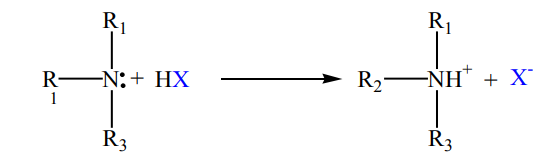
Checking up 9.4
1. Draw the structures of all amines of molecular formula . Classify
. Classify
them as primary, secondary and tertiary amines
2. a. Write the equation for the reaction which happens when dimethylamine,
 reacts with water.
reacts with water.
b. Write the formula of the product of the reaction between trimethylamine
gas, and hydrogen chloride gas, showing the essential details of
and hydrogen chloride gas, showing the essential details of
its structure.
3.Nitrous acid is unstable and has to be produced in the same test tube as the
reaction is happening in. If you were to test an amine with nitrous acid, howwould you do it?
4.This question is about the reactions between amines and halogenoalkanes
(alkylhalides).
The reaction between bromoethane and ethylamine produces a complicated
mixture of products, but the first to be formed are given by the equationsbelow:

Describe in words what is happening.
These next three questions are deliberately made uncooperative so that you
can only do them by understanding what you have read, and not just learning
it. Take your time over them.
5. Write the formulae for the corresponding products of the reaction if you started
with methylamine rather than ethylamine, but still reacted it with bromoethane.
6. If you started with a secondary amine such as dimethylamine, you would
initially get a tertiary amine and its salt in the mixture. Write the formulae of
these products if you reacted dimethylamine with bromoethane.
7. Draw the structure of the product that you would get if you reacted the tertiaryamine trimethylamine with bromoethane.
9.5. General structure of amino acids and some commonexamples
Activity 9.5
Amines are molecules that have as general formula, while carboxylic
while carboxylic
acids have as general formula, R–COOH. Predict a general structure (skeletal
formula) of a molecule that contains an amino group and a carboxyl group onan aliphatic chain.
9.5.1. General structure of amino acids
Amino acids are organic compounds containing amine and carboxyl (-COOH)
and carboxyl (-COOH)
functional groups, along with a side chain (R group) specific to each amino acid.
The key elements of an amino acid are carbon (C), hydrogen (H), oxygen (O), and
nitrogen (N). About 500 naturally occurring amino acids are known.
The general structure of amino acid is shown by the functional group and
a carboxylic acid group (-COOH) attached to the same carbon and they are calledα-amino acids.
The R group is the part of the amino acid that can vary in different amino acids. It
can be a hydrogen (in that case, the amino acid is called Glycine) or a
group (Alanine) or other radicals.
9.5.2. Common Amino Acids
Among the 500 known amino acids, there are 20 important α-amino acids, as shown
in the Table 9.4 below. Each amino acid has a common name. You will notice that
the names in common used for amino acids are not descriptive of their structural
formulas; but at least they have the advantage of being shorter than the systematic
names. The abbreviations (Gly, Glu, …) that are listed in table below, are particularly
useful in designating the sequences of amino acids in proteins and peptides.
Table 9.4: Common amino acids and their formulas [adapted from (Chang, 2005) and(Schmitz, 2018)]




The first amino acid to be isolated was asparagine in 1806. It was obtained from
protein found in asparagus juice (hence the name). Glycine, the major amino acid
found in gelatin, was named for its sweet taste (Greek glykys, meaning “sweet”). In
some cases an amino acid found in a protein is actually a derivative of one of thecommon 20 amino acids.
Checking up 9.5
1. Write the side chain of each amino acid.
a. serine
b. argininec. phenylalanine
2. Draw the structure for each amino acid.
a. alanine
b. cysteine
c. histidine
3. Identify an amino acid whose side chain contains:
a. amide functional group.
b. aromatic ring.c. carboxyl group.
9.6. Comparison of physical properties of amino acids tothose of carboxylic acids and amines
Activity 9.6
By using examples, distinguish amines, carboxylic acids and amino acids (indifferent states: gas, liquid, solid) based on their characteristic odour.
The amino acids, carboxylic acids and amines have different functional groups;
this is the base of their different physical properties as shown in the Table 9.5.Table 9.5. Comparison of physical properties of amines, carboxylic acids and amino
acids.
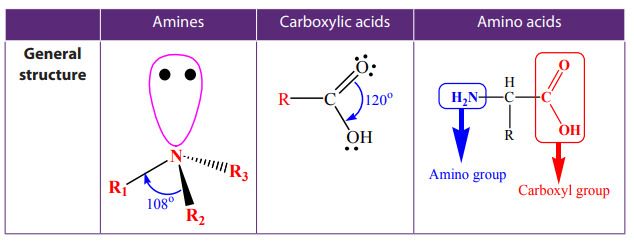
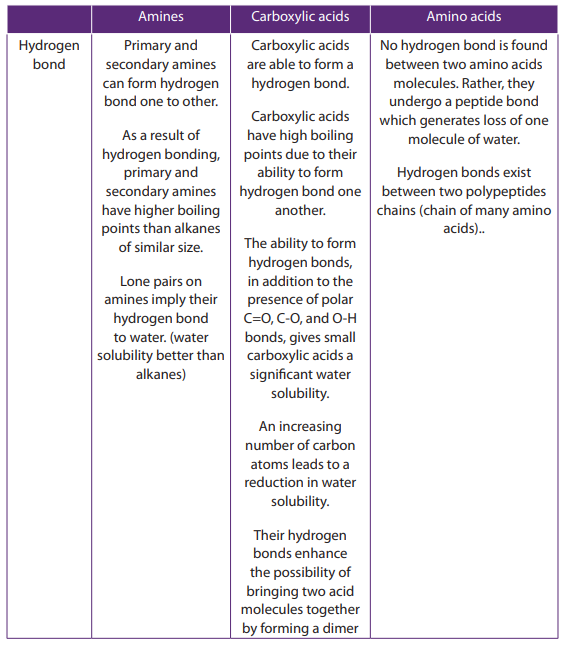
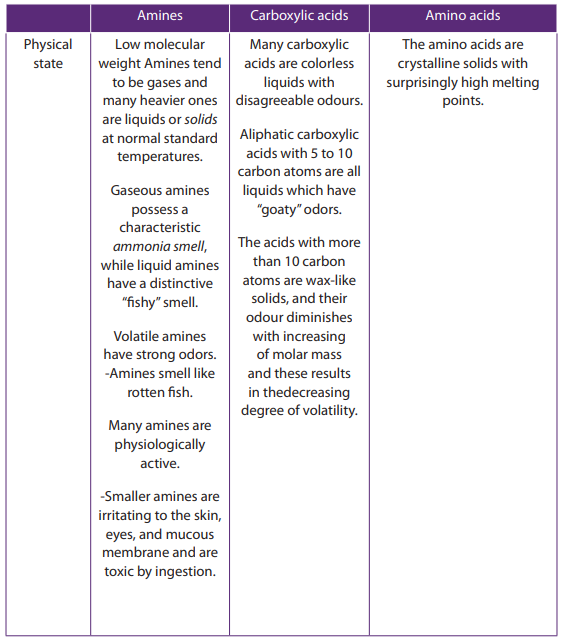

Checking up 9.6
Discuss the solubility of amino acids, referring on the solubility of amines andcarboxylic acids
9.7. Chemical properties of amino acids
Activity 9
A and B are organic products formed from the reactions in a) and b)respectively, propose a mechanism of those reactions and identify A and B.
The reactivity of amino acids involves the reactions of both amines and carboxylic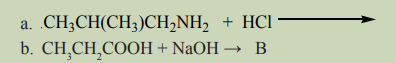
acids. Some of these reactions are given below.
9.7.1. Acid–base properties of amino acids
As the name suggests, amino acids are organic compounds that contain both a
carboxylic acid group and an amine group. Amino acids are crystalline, high melting
point (>200°C) solids. Such high melting points are unusual for a substance with
molecules of this size — they are a result of internal ionisation. Even in the solid
state, amino acids exist as zwitterions in which a proton has been lost from thecarboxyl group and accepted by the nitrogen of the amine group:
So instead of hydrogen bonds between the amino acid molecules there are stronger
ionic (electrovalent) bonds. This is reflected in the relative lack of solubility of amino
acids in non- aqueous solvents compared with their solubility in water.
Zwitterions exhibit acid–base behaviour because they can accept and donate
protons. In acids a proton is accepted by the carboxylic acid anion, forming a unitwith an overall positive charge:
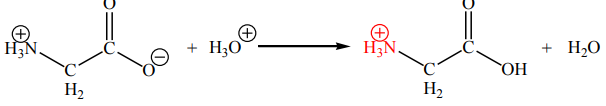
In alkalis the reverse occurs with the loss of a proton from the nitrogen atom:
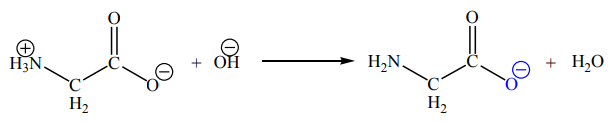
The species present in a given solution depends on the pH of the solution.
Carboxylic acids have acidic properties and react with bases. Amines have basic
properties and react with acids. It therefore follows that amino acids have bothacidic and basic properties.
9.7.2. Isoelectric point in aminoacids (pI)
The isoelectric point (pI), is the pH at which a particular molecule carries no net
electrical charge in the statistical mean. This means it is the pH at which the amino
acid is neutral, i.e. the zwitterion form is dominant. The pI is given by the average ofthe pKa that involve the zwitterion, i.e. that give the boundaries to its existence
The table below shows the pKa values and the isoelectronic point, pI, are givenbelow for the 20 α-amino acids (Table 9.6).

Table 9.6. pKa and pI values for the 20 α-amino acids


There are 3 cases to consider:
1. Neutral side chains
These amino acids are characterised by two pKa values: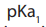 and
and  for the
for the
carboxylic acid and the amine respectively. The isoelectronic point will be halfway
between, or the average of, these two pKa values . This is most readily appreciated
when you realise that at very acidic pH (below pka1) the amino acid will have an
overall positive charge and at very basic pH (above ) the amino acid will have
) the amino acid will have an overall negative charge.
The other two cases introduce other ionisable groups in the side chain “R” described
by a third acid dissociation constant,

2. Acidic side chains
The pI will be at a lower pH because the acidic side chain introduces an “extra”
negative charge. So the neutral form exists under more acidic conditions when the
extra -ve has been neutralised. For example, for aspartic acid shown below, the
neutral form is dominant between pH 1.88 and 3.65, pI is halfway between thesetwo values
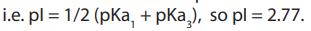
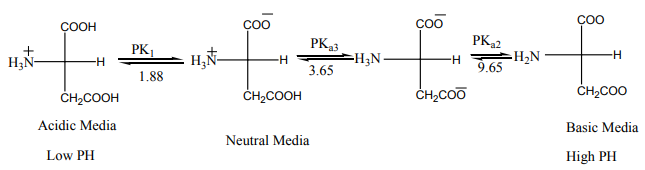
3. Basic side chains
The pI will be at a higher pH because the basic side chain introduces an “extra”
positive charge. So the neutral form exists under more basic conditions when the
extra positive has been neutralised. For example, for histidine, which has three acidic
groups of pKa’s 1.82 (carboxylic acid), 6.04 (pyrrole NH) and 9.17 (ammonium NH),
the neutral form is dominant between pH 6.04 and 9.17; pI is halfway between thesetwo values, i.e. , so pI = 7.60.
9.7.3. Reaction with strong acids
In the following reaction, amino acids react with strong acids such as hydrochloricacid:
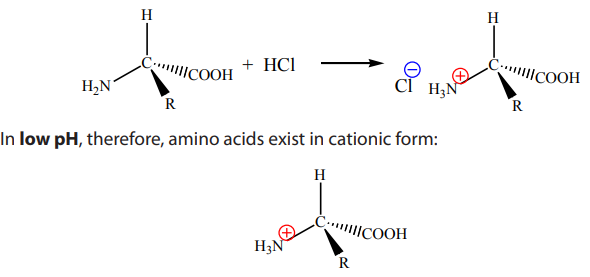
9.7.4. Reaction with nitrous acid (deamination)
The amine function of α-amino acids and esters reacts with nitrous acid in a similar
manner to that described for primary amines. The diazonium ion intermediate loses
molecular nitrogen in the case of the acid, but the diazonium ester loses a protonand forms a relatively stable diazo compound known as ethyl diazo ethanoate:
The diazo ester is formed because of the loss of
 from the diazonium ion which
from the diazonium ion which results in the formation of a quite unfavourable carbocation.
9.7.5. Reaction with sodium hydroxide
Amino acids react with strong bases such as sodium hydroxide:
9.7.5. Reaction with sodium hydroxideAmino acids react with strong bases such as sodium hydroxide:

In high pH, therefore, amino acids exist in anionic form:

9.7.6. Reaction of amino acids with sodium carbonate
Amino acids are instantly dissolved by strong hydrochloric acid but are in part
recovered unchanged on dilution and evaporation. They are not decomposed by
sodium carbonate but are easily decomposed by sodium hydroxide. (Dakin & West,1928).
Checking up 9.7
1. Draw the structure for the anion formed when glycine (at neutral pH)
reacts with a base.
2. Draw the structure for the cation formed when glycine (at neutral pH)reacts with an acid
3. Calculate the Isoelectric point of Glycine?

4. Calculate the Isoelectric point of Lysine?
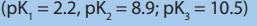
9.8. Optical isomers of amino acids
Activity 9.8
The molecule CHBrClF exhibits optical isomerism. Draw the 3D displayedformulae of both optical isomers.
Simple substances which show optical isomerism exist as two isomers known as
enantiomers. Where the atoms making up the various isomers are joined up in a
different order, this is known as structural isomerism. Structural isomerism is not
a form of stereoisomerism, which involve the atoms of the complex bonded in the
same order, but in different spatial arrangements. Optical isomerism is one form ofstereoisomerism; geometric isomers are a second type.
The general formula for an amino acid (apart from glycine, 2-aminoethanoic acid)
is shown below.The carbon at the centre of the structure has four different groupsattached. In glycine, the “R” group is another hydrogen atom.
The lack of a plane of symmetry means that there will be two stereoisomers of an
amino acid (apart from glycine) - one the non-superimposable mirror image of theother.
For a general 2-amino acid, the isomers are:
The R group, usually referred to as a side chain, determines the properties of each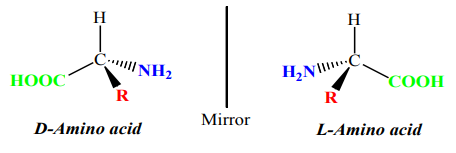
amino acid. Scientists classify amino acids into different categories based on the
nature of the side chain. A tetrahedral carbon atom with four distinct groups is
called chiral. The ability of a molecule to rotate plane polarized light to the left,
L (levorotary) or right, D (dextrorotary) gives it its optical and stereo chemicalfingerprint.
All the naturally occurring amino acids have the right-hand structure in the diagram
above. This is known as the “L-” configuration. The other one is known as the “D-”
configuration.
When asymmetric carbon atoms are present in a molecular compound, there are
two ways in which the groups attached to that carbon can be arranged in the three
dimensions, as we have just shown with the two models above. Chemically, optical
isomers behave in the same way. Biologically, they do not. One will react properly,but the other will not.
Checking up 9.8
1. Write the optical isomers of 2-aminopropanoic and2. Using diagrams, explain why glycine is not chiral.
9.9. Peptides and polypeptides
Activity 9.9
Ethyne is a compound which can undergo a polymerisation reaction.
is a compound which can undergo a polymerisation reaction.
Deduce the reaction of polymerisation of ethyne, showing the mechanism ofreaction.
9.9.1. Formation of peptide bonds
Amino acid molecules can also react with each other; the acidic –COOH group in one
molecule reacts with the basic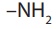 group in another molecule.
group in another molecule.
When two amino acids react together, the resulting molecule is called a dipeptide,
forming an amide linkage (peptide bond), with the elimination of a water molecule.
Each amino acid possesses a carboxylic acid group and an amine group. The
possibilities for constructing polypeptides and proteins are enormous. Let us
consider two simple amino acids, glycine (2-aminoethanoic acid) and alanine(2-aminopropanoic acid).
The figures below show that these can be joined in two ways:
Note: the amide link between the two amino acids. An amide link between two
amino acid molecules is also called a peptide link. The reaction is a condensation
reaction as a small molecule. The dipeptide product still has an
group at one end and a –COOH group at the other end.Therefore the reaction can continue,
to form a tripeptide initially, and then ever-longer chains of amino acids.
The longer molecules become known as polypeptides, and then proteins as they get even
longer sequences of amino acids. A typical protein is formed from between 50 and200 amino acids joined in a variety of sequences.
9.9.2. Structure of peptides and polypetides
A series of amino acids joined by peptide bonds form a polypeptide chain, and each
amino acid unit in a polypeptide is called a residue. A polypeptide chain has polarity
because its ends are different, with α-amino group at one end and α-carboxyl
group at the other. By convention, the amino end is taken to be the beginning of
a polypeptide chain, and so the sequence of amino acids in a polypeptide chain
is written starting with the aminoterminal residue. Thus, in the pentapeptide
TyrGly-Gly-Phe-Leu (YGGFL), tyrosine is the amino-terminal (N-terminal) residue and
leucine is the carboxyl-terminal (C-terminal)residue Leu-Phe-Gly-Gly-Tyr (LFGGY) isa different pentapeptide, with different chemical properties.
This above illustration of the pentapeptide Tyr-Gly-Gly-Phe-Leu (YGGFL) shows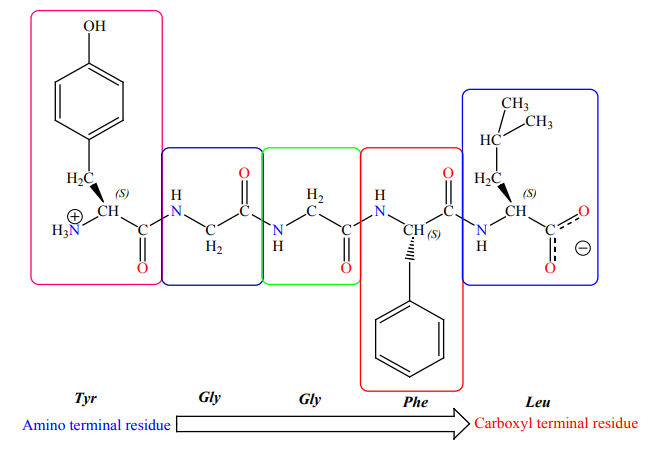
the sequence from the amino terminus to the carboxyl terminus. This pentapeptide,
Leu-enkephalin, is an opioid peptide that modulates the perception of pain. The
reverse pentapeptide, Leu-Phe-Gly-Gly-Tyr (LFGGY), is a different molecule andshows no such effects.
A polypeptide chain consists of a regularly repeating part, called the main chain
or backbone, and a variable part, comprising the distinctive side chains. The
polypeptide backbone is rich in hydrogen-bonding potential. Each residue contains
a carbonyl group, which is a good hydrogen-bond acceptor and, with the exception
of proline, an NH group, which is a good hydrogen-bond donor. These groups
interact with each other and with functional groups from side chains to stabilizeparticular structures, as will be discussed later.
A polypeptide chain consists of a constant backbone (shown in blue) and variable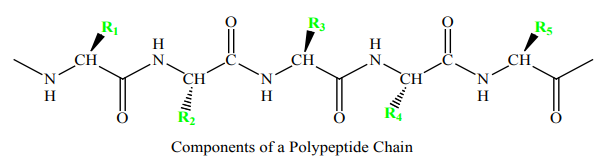
side chains (shown in green).
9.9.3. Uses of amino acids as building blocks of proteins
Like carbohydrates and lipids, proteins contain the elements carbon (C), hydrogen
(H) and oxygen (O), but in addition they also always contain nitrogen (N).sulphur(S)
is often present as well as iron (Fe) and phosphorus (P). Before understanding howproteins are constructed, the structure of amino acids should be noted.
The process of construction of proteins begins by amino acids bonding together, as
seen earlier, through peptide bonds. When many amino acids join together a long
chain polypeptide is produced. The linking of amino acids in this way takes place
during protein synthesis.
The simplest level of protein structure, primary structure, is simply the sequence of
amino acids in a polypeptide chain. The primary structure (Figure 9.2) of a protein
refers to its linear sequence of amino de (–S–S–) bridges. One of those sequences is:–Gly–Ile–Val–Cyst–Glu–Gln–Ala–Ser–Leu–Asp–Arg–Asp–Arg–Cys–Val–Pro–
The primary structure is held together by peptide bonds that are made during the
process of protein biosynthesis. The two ends of the polypeptide chain are referred
to as the carboxyl terminus (C-terminus) and the amino terminus (N-terminus) basedon the nature of the free group on each extremity.
For example, the hormone insulin (Figure 9.3) has two polypeptide chains, A and B,
shown in diagram below. Each chain has its own set of amino acids, assembled in a
particular order. For instance, the sequence of the A chain starts with glycine at the
N-terminus and ends with asparagine at the C-terminus, and is different from the
sequence of the B chain. You may notice that the insulin chains are linked togetherby sulfur-containing bonds between cysteines.
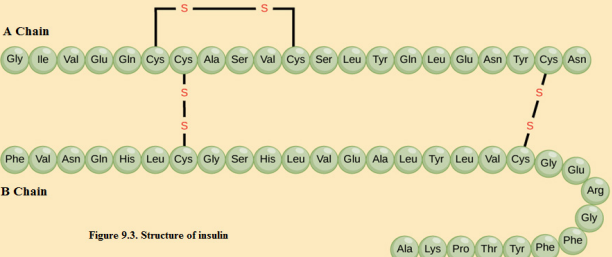
Checking Up 9.9
1. Distinguish between the N– terminal amino acid and the C– terminal
amino acid of a peptide or protein.
2. Describe the difference between an amino acid and a peptide.
3. Amino acid units in a protein are connected by peptide bonds. What is
another name for the functional group linking the amino acids?
4. Draw the structure for each peptide.
a. gly-val
b. val-gly5. Identify the C– and N– terminal amino acids for the peptide lys-val-phegly-arg-cys.
9.10. End Unit Assessment
1. Ethylamine and phenylamine are two organic compounds and both are basic.
a. Draw the displayed formula of each compound, including lone pairs.
b. Write a balanced symbol equation for the reaction between one of these
compounds and an acid to form a salt.
c. Which structural feature of each compound accounts for the basicity?
2. The formulae of two amino acids, glycine (Gly) and alanine (Ala), aregiven:
a. i. Give the systematic names of both amino acids.
ii. Draw their skeletal formulae.
b. Alanine can exist as two stereoisomers.
i. Draw these two stereoisomers, showing how they differ in their spatial
arrangements.ii. Explain why glycine does not have stereoisomers.
3. The structure of a certain tripeptide is shown here:
a. i. Draw the displayed formulae of the three amino acids that make up the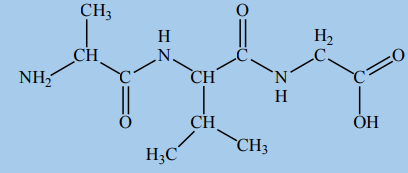
tripeptide.
ii. Which of these amino acids has two chiral carbon atoms?
b. This tripeptide can be split up into the three amino acids by refluxing with
aqueous hydrochloric acid.
i. Which bond is broken in this reaction?
ii. The reaction can be described as hydrolysis. Explain why, using adiagram.
UNIT 10. PHASE DIAGRAMS
Key unit competency:
To be able to interpret the phase diagrams for different compounds.
• Define a phase
• Explain the term phase equilibrium
• Explain the effect of change of state on changing pressure and temperature
• Define heterogeneous and homogeneous equilibria
• Define triple point, critical point, normal boiling and melting points of
substances
• Relate the physical properties of compounds to their phase diagrams.
• Locate triple point, critical point, normal boiling and melting points on the
phase
• diagrams
• Compare the phase diagrams for water with that carbon dioxide
• Develop analysis skills, team work, and attentiveness in interpreting thephase diagrams and in practical activities
Introductory Activity
In our daily life, we use varied materials which have distinct properties and they
can exist in different physical states of the matter. In this regard, we sometimes
need to keep a given substance in a certain state because we know that it will
serve better. Explain two important conditions that should be dealt with to
maintain stable some physical states of the matter as we need them.Explain why ice flots on water.
10.1. Phase equilibrium
Activity 10.1
1. Observe the diagrams that represent different systems and assess thenumber of phases and components involved in each system.

2. Differentiate a phase and a physical state of matter. Explain your answer.
10.1.1. Definition of key terms
A phase is a homogeneous portion of a system which has uniform physical
characteristics. It can be separated from other parts of the system by a clear boundary
(limit). A phase can be a solid, liquid, vapor (gas) or aqueous solution which is uniformin both chemical composition and physical state.
Examples
i. A mixture of gases (air) consists of one phase only
ii. A mixture of oil and water consists of two different liquid phasesiii. A mixture of solids, each solid is regarded as having one phase
A component: it is a chemical species which may be used to specify the
composition of a system. For example;
• A three-phase system of water (i.e. water, ice, and vapor) is a one component
system. The constituent substance of the three phases is water only.
• A mixture of water and ethanol is a one phase, two components system
because there are two different chemical compositions.
• An equilibrium: it is the state of a reaction or physical change in which the
rates of the forward and reverse processes are the same and there is no net
change on the amount of the equilibrium components
• A phase equilibrium: it is a balance between phases, that is, the coexistence
of two or more phases in a state of dynamic equilibrium. The forward process
is taking place at the same rate as the backward process and therefore the
relative quantity of each phase remains unchanged unless the externalcondition is altered.

Checking up 10.1
1. Which of the following is not an example of phase equilibrium?

b) Carbon dioxide in a stoppered fizzy drink:
c) Vapours above the surface of liquid water in a closed container, at a given
temperature.
2. At 0.001°C and 0.00603 atm water, ice and vapor can coexist in a closed
container.
a) Explain the number of phases that this equilibrium has.b) How many components does this system have? Explain.
10.2. Homogeneous and heterogeneous equilibria
Activity 10.2
Differentiate homogeneous mixture from heterogeneous mixture. mixture
of solids, each solid is regarded as having one phase
1. Homogeneous equilibrium
A system with one phase only is described as a homogeneous system and when
this system is at equilibrium, it is said to be a homogenous equilibrium.
In general, a homogeneous equilibrium is one in which all components are present
in a single phase. In a case of a chemical reaction, both reactants and products exist
in one phase (gaseous phase, liquid phase or aqueous solution and solid phase).
For example, in the esterification of acetic acid and ethanol the equilibrium is
homogeneous because all involved substances are in the same liquid phase.
All the reactants and products are liquids
2. Heterogeneous equilibrium
A system consisting of more than one distinct phases is described as heterogeneous
system. A heterogeneous equilibrium is a system in which the constituents are
found in two or more phases. The phases may be any combination of solid, liquid,
gas, or solutions.
For example, in the manufacture of quick lime from lime
stone the following equilibrium is involved:
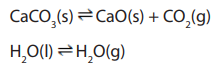
It is a heterogeneous equilibrium because some of the components are solids (limestone and quick lime) and another is a gas (carbon dioxide).
Checking up 10.2
Classify the following reactions as homogeneous equilibrium and heterogeneousequilibrium

10.3. Phase diagrams
Activity 10.3
1. When ice cream trucks drive through towns on hot season days, they
keep their products from melting by using dry ice (solid carbon dioxide)as shown in the image below. Why is dry ice used instead of ice?

2. Why most of the time very high mountains are covered by ice?
3. Explain the conditions that are required to be changed so that the pure
substance change from one state of matter to another.
Aphase diagram is a graph illustrating the conditions of temperature and pressure
under which equilibrium exists between the distinct phases (states of matter) of a
substance. Phase diagrams are divided into three single phase regions that cover
the pressure-temperature space over which the matter being evaluated exists:
liquid, gaseous, and solid states. The lines that separate these single-phase regions
are known as phase boundaries. Along the phase boundaries, the matter being
evaluated exists simultaneously in equilibrium between the two states that borderthe phase boundary.
The general form of a phase diagram for a substance that exhibits three phasesis shown below in the Figure 10.1.

Under appropriate conditions of temperature and pressure of a solid can be in
equilibrium with its liquid state or even with its gaseous state. The phase diagram
allows to predict the phase of substance that is stable at any given temperature and
pressure. It contains three important curves, each of which represents the conditionsof temperature and pressure at which the various phases can coexist at equilibrium.
i. Boiling point
The line TC is the vapor pressure curve of the liquid. It represents the equilibrium
between the liquid and the gas phases. The temperature on this curve where the
vapor pressure is equal to 1atm and it is the normal boiling point of the substance.
The vapor pressure curve ends at the critical point (C) which is the critical
temperature corresponding to the critical pressure of the substance which is thepressure required to bring about liquefaction at critical temperature.
ii. Critical point
Critical point consists of the temperature and pressure beyond which the liquid and
gas phases cannot be distinguished. Every substance has a critical temperatureabove which the gas cannot be liquefied, regardless the applied pressure.
iii. Sublimation point
The line AT is the sublimation curve which represent the variation in the vapor
pressure of the solid as it sublimes into gas at different temperatures. The reverse
process is deposition of the gas as a solid. Sublimation point is the temperature atwhich the solid turns to gas at a constant pressure.
iv. Melting point
The line TB is the melting point curve which represent the change in melting point
of the solid with increasing pressure.The line usually slopes slightly to the right as
pressure increases. For most substances, the solid is denser than the liquid, therefore,
an increase in pressure favors the more compact solid. Thus, higher temperatures
are required to melt the solid at higher pressures. The temperature at which thesolid melts at a pressure of 1atm is the “normal melting point”.
v. Triple point
The triple point T is a point where the three curves intersect. All the three phases
exist at equilibrium at this temperature and pressure. The triple point is unique foreach substance.
vi. Supercritical fluid
Supercritical fluid of a substance is the temperature and pressure above its own
thermodynamic critical point that can diffuse through solids like a gas and dissolvedmaterials like a liquid.
Any point on the diagram that does not fall on a line corresponds to conditions
under which one phase is present. Any other point on the three curves represents
equilibrium between two phases.
The gas phase is stable phase at low pressures and elevated temperatures. The
conditions under which the solid phase is stable extend to low temperatures and
high pressures. The stability range for liquids lie between the other two regions. Thatis between solid and liquid regions.
10.3. 1. Phase diagram of water
Water is a unique substance in many ways due to its properties. One of these special
properties is the fact that solid water (ice) is less dense than liquid water just abovethe freezing point. The phase diagram for water is shown in the Figure 10.2.
Water can turn into vapor at any temperature that falls on the vapor pressure curve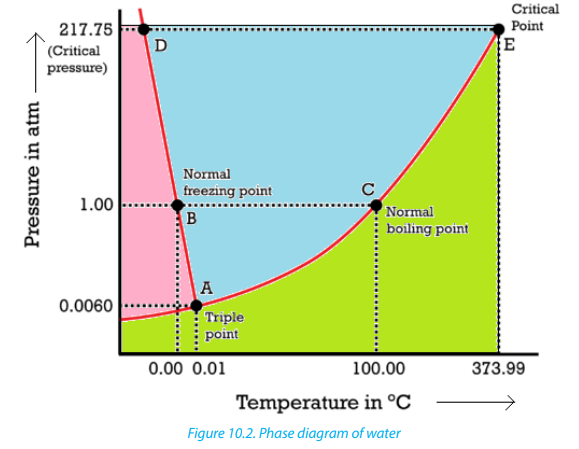
depending on the conditions of pressure, but the temperature at which water liquid
turns into vapor at normal pressure (1atm) is called the normal boiling point ofwater, 100 °C (Figure 10.2)
Point E in the Figure 10.2 is the critical point of water where the pressure is equal
to 218 atm and the temperature is about 374 °C. At 374°C, particles of water in the
gas phase are moving rapidly.At any other temperature above the critical point of
water, the physical nature of water liquid and steam cannot be distinguished; the
gas phase cannot be made to liquefy, no matter how much pressure is applied tothe gas.
The phase diagram of water is not a typical example of a one component system
because the line AD (melting point curve) slopes upward from right to left. It has a
negative slope and its melting point decreases as the pressure increases. This occurs
only for substances that expand on freezing. Therefore, liquid water is denser thansolid water (ice), the reason why ice floats on water.
10.3.2. Phase diagram of carbon dioxide
Compared to the phase diagram of water, in the phase diagram of carbon dioxide
the solid-liquid curve exhibits a positive slope, indicating that the melting point for increases with pressure as it does for most substances. The increase of pressure
increases with pressure as it does for most substances. The increase of pressure
causes the equilibrium between dry ice and carbon dioxide liquid to shift in the
direction of formation of dry ice that is freezing. Carbon dioxide contracts on freezing
and this implies that dry ice has higher density than that of liquid carbon dioxide.The Figure 10.3 shows the phase diagram of carbon dioxide.

The triple point is observed at the pressure above 1atm, indicating that carbon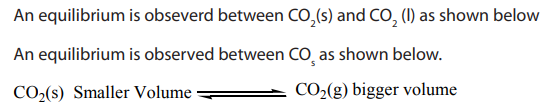
dioxide cannot exist as a liquid under normal conditions of pressure. Instead,
cooling gaseous carbon dioxide at 1atm results in its deposition into the solid state.
Likewise, solid carbon dioxide does not melt at 1atm pressure but instead sublimesto yield gaseous

Checking up 10.3
1. If a piece of dry ice is left on the lab counter, you will see it get smaller
until it disappears, with no liquid left around it. Explain why.
2. Describe what conditions of pressure and temperature will carbon
dioxide exist as a liquid?
3. What is the meaning of the term “critical temperature”, and what is the
value of the critical temperature of ?
?
4. Why does make an excellent fire extinguisher?
make an excellent fire extinguisher?
5. Explain the following observations:
a) When a closed glass container full of water is put in fridge, it directly
breaks when the water freezes.
b) The water of oceans at the poles of the Earth are normally covered byice and ice does not submerge in water.
10.4. Comparison of phase diagrams of substances that
expand and those that contract on freezing
Activity 10.4
1. Analyze the phase diagrams of water and carbon dioxide previously
discussed to assess their similarities and differences.
2. The glacier easily slides on ice as shown in the photo below. Explain howthe property of water facilitates this movement.

For the phase diagrams, some materials contract on freezing while others expand
on freezing. The main differences between substances that expand and those
that contract on freezing can be highlighted by comparing the phase diagrams
of carbon dioxide and that of water. In the phase diagram of carbon dioxide, thesubstance contracts on freezing and that of water expands on freezing.
Both phase diagrams for water and carbon dioxide have the same general Y-shape,
just shifted relative to one another. This shift occurs because the liquid phase in
the dry ice can only occur at higher temperatures and pressures, whereas, in ice
the liquid phase occurs at lower temperatures and pressures. There are two more
significant differences between the phase diagram of carbon dioxide and that ofwater:
10.4.1. Melting point curve
The melting point curve of carbon dioxide slopes upwards to right (Figure 10.3)
whereas that of water slopes upward to left (Figure 10.2). This means that for carbon
dioxide the melting point increases as the pressure increases, a characteristic
behavior of substances that contract on freezing. Further, water expands on freezing
(Figure 1.4) and this unusual behavior is caused by the open structure of the regular
packing of water molecules in ice due to the network of hydrogen bonding in icewhich is more extensive than in liquid.
Ice floats on liquid water (Figure 10.5), this unusual behavior is caused by the open
structure of the regular packing of water molecules in ice due to the network of
hydrogen bonding in ice which is more extensive than in liquid. The ice is less densethan water reason why it floats in water.

10.4.2. Triple point
The triple point of carbon dioxide is above atmospheric pressure. This means that
the state of liquid carbon dioxide does not exist at ordinary atmospheric pressure.
Dry ice remains as a solid below -78ºC and changes to fog (gas) above -78ºC. It
sublimes without forming liquid at normal atmospheric pressure (Figure 10.6). The
sublimation of carbon dioxide results in a low temperature which causes watervapors in the air to form moist.
Ice is stable below 0 ºC and water is stable between 0ºC and 100 ºC while water
vapor is stable above 100 ºC. At normal atmospheric pressure, ice can first melts andultimately boils as the temperature increases.
Checking up 10. 4
1. Explain three ways that dry ice is different to the normal ice.
2. Explain why the liquid phase is not observed in the dry ice as it sublimes,
whereas all three phases are observed in the ice?
3. At temperature and pressure of 5ºC and 1atm (refer to both phase
diagram of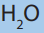 and
and 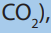 are normal ice and dry ice at the same phase?
are normal ice and dry ice at the same phase?
Explain your reasoning.
4. Draw and label a phase diagram for water and carbon dioxide and
explain why they are different?5. Explain the reason why a glass container breaks when water freezes.
10.5. Applied aspect of phase diagrams
Activity 10.5
Engineers use diverse materials in construction of houses, bridges, etc. and in
making different other products such as cars, airplanes, computers, etc. Explain
if the knowledge of the phase diagrams of those materials the engineers use isimportant to them.
The applications of phase diagrams are useful for engineer’s materials and material
applications. The scientists and engineers understand the behavior of a system
which may contain more than one component. Multicomponent phase’s diagramsshow the conditions for the formation of solutions and new compounds.
The phase diagrams are applied in solidification and casting problems. Many materials
and alloy system exist in more than one phase depending on the conditions of
temperature, pressure and compositions. In the area of alloy development, phase
diagrams have proved invaluable for tailoring existing alloys to avoid over design
in current applications, each phase has different microstructure which is related
to mechanical properties. The development of microstructure is related to the
characteristics of phase diagrams. Proper knowledge and understanding of phase
diagrams lead to the design and control of heating procedures for developing therequired microstructure and properties.
Phase diagrams are consulted when materials are attacked by corrosion. They
predict the temperature at which freezing or melting begins or ends. Phase
diagrams differentiate the critical point, triple point, normal boiling point, etc ofsome substances.
Examples
• Zn-Fe based high-order phase diagrams have found a wide range of
applications in continuous galvanizing.
• The Zn-rich corner of the Zn-Fe-Al phase diagram is being used daily forscientific interpretation of bath assays.
In general the industrial applications of phase diagrams include alloy design,processing, and performance.
Checking up 10.5Do research and explain different applications of phase diagrams.
10.6. End unit assessment
1. At pressures lower than triple point, water cannot exist as liquid,
regardless of the temperature.
a. True b) False
2. The melting point of water decreases as the pressure is augmented
because water contracts on freezing.
a. True b) False
3. The melting point of carbon dioxide increases as the pressure is raised
because carbon dioxide expands on freezing.
a). True b). False
4. Use the following phase diagram of water to answer the questions
related:
a. At a pressure of 1atmosphere, what is the normal freezing point of
water?
b. What is the normal boiling point of water, at 1atmosphere of water?
c. In Karisimbi, we live approximately 5,500 feet above sea level, which
means the normal atmospheric pressure is less than 1atm. In Karisimbi,
will water freeze at a lower temperature or a higher temperature than
at 1atmosphere?
d. Will water boil at a higher or lower temperature, than at 1atmosphere?
5. If we shake a carbon dioxide fire extinguisher on a cool day , we
can hear liquid sloshing around inside the cylinder. However, the
sloshing around inside the cylinder. However, the
cylinder appears to contain no liquid on a sweltering day,
Explain these observations.6. Observe the diagram below and answer the related question.
a. Explain what is labeled in the parts X, Y, Z, C and T
b. Would the substance represented on this graph contract or expand when
it was frozen? Explain your answer.
c. Describe what will happen to Y if the temperature is increased at constant
pressure.
d. Explain what will happen to X if the pressure is much lowered at constant
temperature.
7. The diagram below shows the variation of vapor pressure with temperaturefor pure substance.
a. What sections represent liquid, gas, solid phases?
b. What letter represents the triple point? Give the definition of the triple
point.
c. What is the substance‘s normal boiling and melting point?
d. Above which temperature it is not possible to liquefy the gas of the
substance, no matter how much pressure is applied?
e. At a constant temperature, what would you do to cause this substanceto change from the liquid phase to the solid phase?
UNIT 11: SOLUTIONS AND TITRATION
Key unit competency:
Be able to prepare standard solutions and use them to determine concentration ofother solutions by titration.
Learning objectives:
• Define the terms standard solution and primary standard solution.
• Explain the properties of a standard primary solution.
• Explain the titration process, emphasising the need for precise measurements.
• Prepare solutions with different concentrations.
• Properly use the burettes, pipettes during titration.
• Interpret the experimental data obtained by titration and report.
• Carry out acid-base, redox titrations and do calculations involved.
• Develop a team approach and a sense of responsibility in performing the
experiments of titration.
• Respect of procedure in practical experiment.
• Develop a culture of orderliness in performing practical experiments.• Appreciate the use of appropriate measurements in daily life.
Introductory Activity

Observe the above photo and attempt the following questions:
1. For the bottle of PRIMUS, and that of MUTZIG, we find on the bottle of its
labels 5% alcohol and 5.5% alcohol respectively.
a. Explain the meaning of 5% and 5.5% alcohol.
b. 5% alcohol corresponds to which volume of alcohol of the total
volume of 72cl of the bottle of PRIMUS.
c. Calculate the volume of alcohol corresponding to 5.5% alcohol of the
bottle of MUTZIG of the total volume of 65cl.
2 . On the label of the bottle of AGASHYA JUICE we find that dilution is 1:5.Explain this ratio and explain also the purpose of diluting substances.
11.1. Definition of standard solution and primary standard solution.
Activity 11.1
1. You have a bag full of rice. How do you determine its weigth?
2. You have a jerrycan half-full of a liquid substance, how are you going to
proceed to know the exact quantity of the liquid?
3. You are given a basic solution, NaOH (aq), and you are requested todetermine its concentration. What do you need to do that?
In analytical chemistry, a standard solution is a solution containing a precisely known
concentration of an element or a substance and used to determine the unknown
concentration of other solutions. A known weight of solute is dissolved to make a
specific volume. It is prepared using a standard substance, such as a primary standard.
A primary standard is defined as a substance or compound used to prepare standard
solutions by actually weighing a known mass, dissolving it, and diluting to a definitevolume.
Or a substance, which is chemically stable in aqueous solution and its concentration
remains constant with change in time such that it can be used to standardize other
solutions.
Some important examples of primary standard are;

Standard solutions are normally used in titrations to determine unknown concentrationof another substance.
Checking up 11.1Differentiate between standard solution and primary standard solution
11.2. Properties of a primary standard solution.
Activity 11.2
Do research and find out the role and characteristics of a good primary standard.
A good primary standard meets the following criteria:
• High level of purity
• High stability
• Be readily soluble in water
• High equivalent weight (to reduce error from mass measurements)
• Not hygroscopic (to reduce changes in mass in humid versus dry environments)
• Non-toxic
• Inexpensive and readily available
• React instantaneously, stoichiometrically and irreversibly with other substances
i.e. should not have interfering products during titration.
• It should not get affected by carbon dioxide in air
Molar concentrations are the most useful in chemical reaction calculations because
they directly relate the moles of solute to the volume of solution. The formula formolarity is:
Checking up 11.2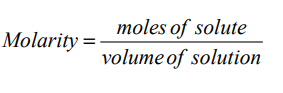
Discuss the properties of a good primary standard
11.3. Preparation of standard solutions
Activity 11.3
Describe how you would prepare 1L of a 1M solution of sodium chloride. The gramformula weight of sodium chloride is 58.5g/mol
The preparation of the solution requires a reagent that is so to say the quantity to be
weighed for mass if the reagent is in solid state ; The volume to be pipetted using
pipette if the solute is a liquid and then after dissolve it in water, So the solution can beprepared by two methods such as dissolution method and dilution method.
In the preparation of solution, glasses, volumetric flask, pipette, glass rod, measuring
cylinder, analytical balance, spatula, beakers, magnetic stirrer and other laboratorydevices are used.
11.3.1. Preparation of standard solution by dissolution of solids
Activity 11.3.1
Preparation of 250 mL of 1M NaOH solution
Materials
• Beaker - balance - sodium hydroxide solid
• Spatula - funnel - glass rod• Volumetric flask - stopper - distilled water
Procedure:
i. Calculate the mass of sodium hydroxide needed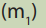
ii. Weigh a clean beaker, and record its mass with a clean spatula, add
a clean spatula, add
sodium hydroxide until the combined mass of weighing beaker and
sodium hydroxide is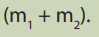
iii. Add about 50 cm3of distilled water, stir with a glass rod until all solids
have dissolved.
iv. Pour all the solution carefully through a funnel into a volumetric flask;
wash all the solution out of the beaker and off the glass rod.
v. Add distilled water until the level is about below the graduation
below the graduation
mark on the graduated flask. Add the rest of distilled water from a washing
bottle until the bottom of the meniscus is at the level of mark when viewed
at eye level.
vi. Insert the stopper of the flask and invert the flask several times to mix the
solution.
vii. Label the solution.
Scope: This method is applied for solute in solid state and you should be able to
determine the mass required from calculation to be weighed and provide distilledwater to dissolve the solute.
Examples:1. Describe in details how you can prepare the following solution: 50 mL of NaOH, 10%.
Answer:
10% means that in 100 mL of solution only 10 g are pure in NaOH, So 50 mL of NaOH
will be prepared by taking the mass of NaOH, dissolving it in water and madding up to50 ml.
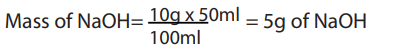
Procedure:
• Weigh 5g of NaOH accurately using glass watch, spatula and analytical balance.
• Dissolve it in a volumetric flask of 50 mL containing already little water and mix
using a baguette and shake till you get homogeneous mixture (you should take
care since it is an exothermic reaction).
• Top up using distilled water and shake again and cover your solution.• Label your solution: 10% NaOH; 50 mL and the date of preparation.
2. Describe in details how you can prepare
 solution.
solution.Answer:
Step 1: CalculationsCalculate the amount of anhydrous sodium carbonate required to be dissolved in
 of solution. i.e.:
of solution. i.e.:
Step 2: Weighing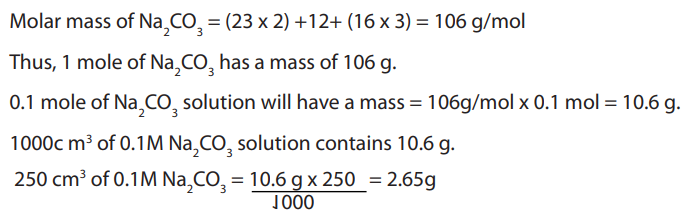
• Weigh a clean empty beaker and record its mass,
• Using a clean spatula add the mass of pure anhydrous sodium carbonate equal
to the calculated mass. Let it be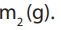
• Actual mass of the carbonate transferred into the beaker =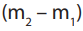 g
g
Note: Not the entire sample gets transferred into the beaker since part of it sticks onthe walls of the weighing bottle.
Step 3: Procedure
• Using a wash bottle, carefully add about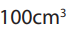 of distilled water. Stir using a
of distilled water. Stir using a
glass rod until the entire solid has dissolved.
• Carefully pour all the solution through a filter funnel into a volumetric flask. Wash
off all the solution out of the beaker and off the glass rod; ensure that all the
washings run into the volumetric flask.
• Add distilled water until the level of the volumetric is about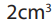
below the mark. Add the rest of the distilled water drop by drop using
mark. Add the rest of the distilled water drop by drop using
a dropping pipette until the bottom of the meniscus is level with the 250cm3
mark when viewed at eye level.
• Insert the stopper of the flask and invert the flask several times to mix the solution.
Note: Since the concentration of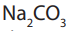 solution has been determined, thus the
solution has been determined, thus the solution prepared is a standard solution.
Checking up 11.3.1
Describe in details, how you can prepare the following solutions:
a.
b. 250 mL acidified potassium dichromate 1Nc. 100 mL oxalic acid 5g/L.
11.3.2. Preparation of standard solution by dilution
Activity 11.3.2
Materials
• Measuring cylinder
• Volumetric flask
• Stopper
• Concentrated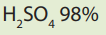
• Distilled water
Notice: Take care when mixing water and acid. When you mix acid with water, it is
extremely important to add the acid to the water rather than the other way around.
It is because acid and water react in a vigorous exothermic reaction, releasing heat,
sometimes boiling the liquid. If you add acid to water, the water is unlikely to splash
up, but even if it did, it is less likely to hurt you than if you add water to acid. Whenwater is added to acid, the water boils and the acid may splatter and splash.
Apart from, dissolution method, we can prepare a solution by dilution from the stock
solution. This consists of reducing the concentration of a concentrated stock solution
to less concentrated solution by adding water.
The rationale is how we can prepare the desired solution of concentration C, with the
volume V, from the stock solution whose concentration is .The question consists of
.The question consists of
determination of the volume to be pipetted from stock solution and diluted using .
to be pipetted from stock solution and diluted using .
Xml of water to get V. So, from the conservation principle of matter, the quantity of thesolute before and after dilution should be the same.
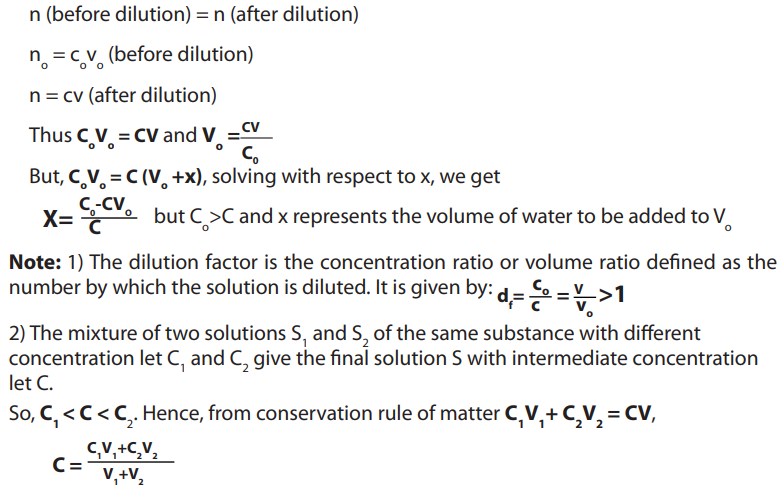

Procedure: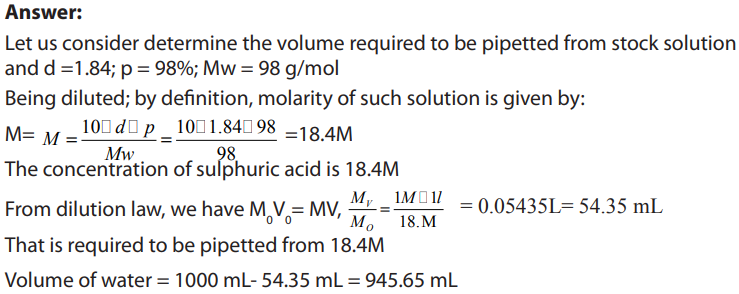
• Pipette only 54.35 ml of sulphuric acid accurately using a pipette from a stock
solution.
• Pour them gently in the flatted balloon of 1L containing already little water and
shake. Note that you should take much care since the reaction is exothermic
and sulphuric acid is harmful to skin remember that we pour acid to water
not water to acid that is A-W not W-A.
• Top up to 1L using distilled water and shake again in order to homogenize the
solution.
• Cover the solution, then label it: and date of preparation.
and date of preparation.2. Calculate the volume of
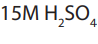 that would be required to prepare
that would be required to prepare 

Checking up 11.3.2
A 0.2N solution was diluted by addition of 200 mL of water.
Calculate the dilution factor if the solution is diluted from 0.2N to 0.05N.Determine the final volume of the solution after dilution.
11.4. Simple acid-base titrations
Activity 11.4
You are provided with

Materials
- Burette - Indicator (phenolphthalein)
- Measuring cylinder - Washing bottle
- Conical flask - Beakers- Retort stand - Funnel
Procedure
a. Using a pipette, transfer 10 mL of into a conical flask.
into a conical flask.
b. Add three drops of phenolphthalein indicator and titrate it with
from the burette.
c. Repeat the titration until you obtain consistent values.d. Record your readings in the table below:
a. Calculate the average volume of
 used.
used.
b. Calculate the number of moles of HCl that react with
c. Calculate the molarity of in 10 mL.
in 10 mL.
Titration is the controlled addition and measurement of the amount of a solution
of known concentration required to react completely with a measured amount of asolution of unknown concentration.
Acid-base titration
It is the determination of the concentration of an acid or base by exactly neutralizingthe acid or base of known concentration
Alkalimetry and acidimetry
• Alkalimetry is the specialized analytic use of acid-base titration to determine
the concentration of a basic substance.
• Acidimetry is the same concept of specialized analytic use of acid-base titrationto determine the concentration of an acidic substance.
The point at which the two solutions used in a titration are present in chemically
Equivalence point
equivalent amount is the equivalence point. At this point the moles of two solutionswill be equal.
Indicators and pH-meters can be used to determine the equivalence point. The
point in a titration at which an indicator changes color is called the end-point of thetitration.
Equipment's and set up (Figure 11.1) of materials for Titration
The common equipment used in a titration are:
• Burette
• Pipette
• pH-indicator/acid-base indicator
• White tile: used to see a color change in the solution(a white paper can also be
used)
• Conical flask (Erlenmeyer flask)
• Titrant: a standard solution of known concentration• Analyte: a solution of unknown concentration

How to perform titrations
Knowing the use of pipette and burettes and how to handle them, the following pointsare useful in order for a correct titration to be done:
1. The apparatus should be arranged as shown in the above Figure.
2. The burette tap is opened with the left hand and the right hand is used to
shake the conical flask.
3. The equivalence-point is reached when the indicator just changes permanently
the colour.
4. At the end point, the level of the titrant is read on the burette
5. The titration is now repeated, three more times are recommended. Towards
the end-point, the titrant is added dropwise to avoid overshooting.
Notice: Before titration, check if the tip of the burette is filled with the titrant, and doesn’t
contain bulb of air. If there is a bulb of air, a quick opening and closing of the tap will expelthe air out of the burette.
Choice of indicators in acid-base titrations
When the technique of acid-base titration is extended to a wide variety of acidic and
alkaline solution, care needs to be taken about the choice of indicator for any givenreaction.
The choice of an inappropriate indicator would lead to incorrect results, and it is
therefore extremely important that the indicator is chosen carefully.
The principle on which a choice of indicator is made concerns the strength of the acid
or base involved in the reaction. Note that the strength of an acid or base is not to be
confused with the concentration of its solution. Example of strong and weak acids andbases and choice of indicator are given in the Table below.
Table 11.1. Examples of strong/weak acids and bases
Indicators which are suitable for particular types of acid-base titrations are given in
the Table 11.2.
Table 11.2. Examples of indicators
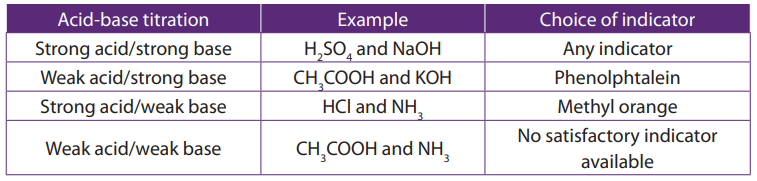
The results are summarized in a table as shown below.
Table 11.3. Sample results
Notice: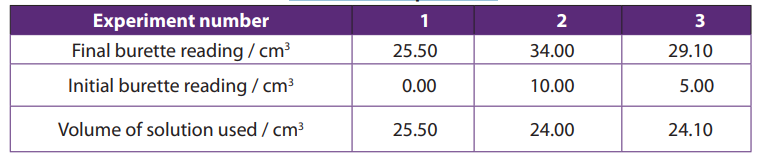
• Burette readings should be written to two decimal places (for burette havingprecision up to hundredth)
Average title should be obtained using values which differ not by more
. Consistent values :24.00 and 24.10
Average volume
Examples: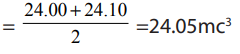
1) Suppose 20.00 mL of NaOH is required to reach the end-point in the
NaOH is required to reach the end-point in the
titration of 10.0 mL of HCl of unknown concentration. How can these titration databe used to determine the molarity of the acidic solution.
Answer:
Begin with the balanced neutralization reaction equation. From the equation,determine the chemically equivalent amount of HCl and NaOH.

Calculating the number of moles of NaOH used in the titration;
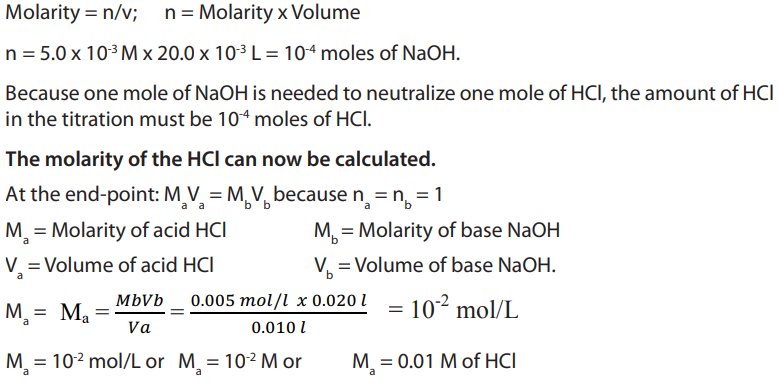

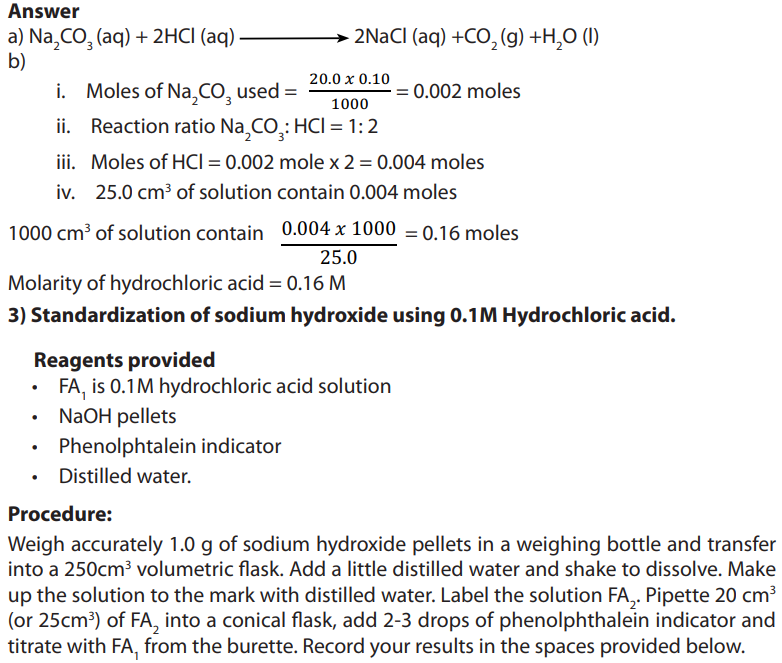
Table 11.4. Burette readings
Each value or entry in the table must be recorded or written to two decimal places.
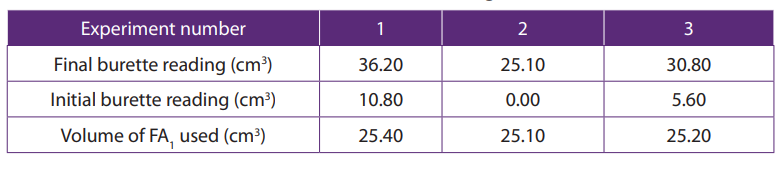
Different initial readings should be used. Initial reading in each experiment should becorrectly subtracted from the final reading.
Questions:

Answer:
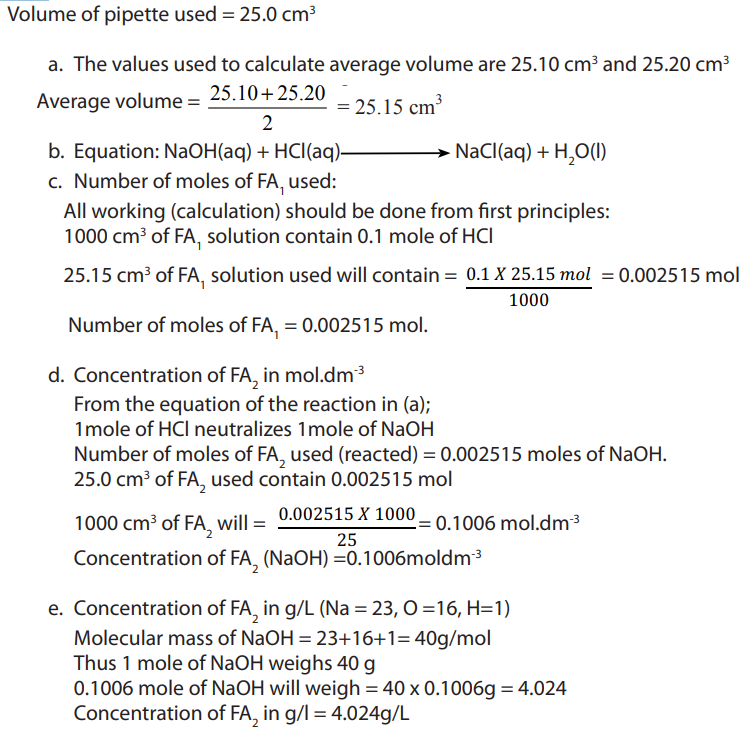


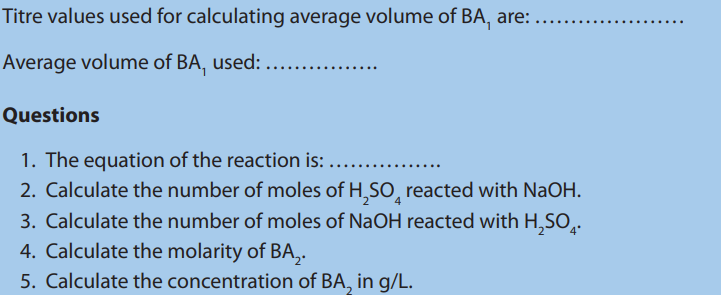
11.5. Titration involving redox reactions
Activity 11.5
1. What does differentiate redox reactions from other reactions?2. Balance the following redox equations using half-equation method:
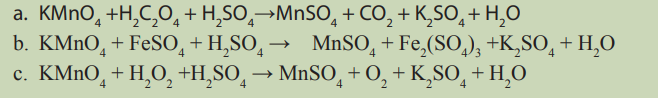
11.5.1. Titrations with potassium manganate (VII);
Potassium manganate (VII),
 is a useful oxidizing agent in a sufficiently acidic
is a useful oxidizing agent in a sufficiently acidic
medium normally used in the reactions such as:
• Oxidation of iron (II) to iron III
• Oxidation of ethanedioate (oxalates) or oxalic acid to carbon dioxide.
• Oxidation of nitrites to nitrates
• Oxidation of hydrogen peroxide to oxygen, etc.
During the reactions, the manganate (VII) ions, which is purple, is reduced to
which is purple, is reduced to
manganese(II), which is pale pink but practically colorless.
which is pale pink but practically colorless.
Thus, titrations involving potassium manganate (VII), do not require an indicator.
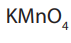 acts as its own indicator.
acts as its own indicator.
The end-point of titration is detected when the solution shows a permanent faint pink
color, which is as a result of slightly excess of potassium manganate (VII).
For reduction of the potassium manganate (VII) to be complete, sufficient acid should
be used and the solution added slowly, not rapidly. Use of insufficient acid results in
precipitation of manganese (IV) oxide, which appears as a brown precipitate.
which appears as a brown precipitate.
The suitable acid for this process is sulphuric acid. Hydrochloric acid is unsuitable
because the ion is a stronger oxidizing agent than
ion is a stronger oxidizing agent than  it oxidizes the chloride
it oxidizes the chloride ion in the acid to molecular chlorine.
Nitric acid is also not used to acidify the solution of potassium manganate (VII) since it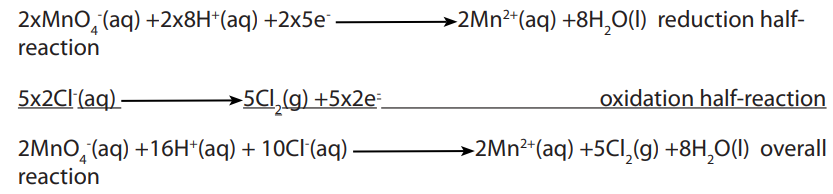
is also a powerful oxidizing agent. In potassium manganate (VII) titrations, an indicator
is used only when the reducing agent forms a colorless solution which makes it difficult
to observe the pink color.
11.5.1. Titration of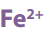 by Potassium manganate (VII),
by Potassium manganate (VII),
Ativity 11.5.1Balance the following redox equations using half-equations method:
Most redox titration labs utilize an iron (II) salt, frequently iron (II) sulphate
heptahydrate. This compound is used for two reasons. First, it has a large molar mass,
making it a good (but not the best) primary standard. Second, its solution is near
colorless, so the pink/purple endpoint is easy to detect. Color proves to be an issue
when dealing with many other compounds. Iron (II) salts are an excellent choice for atitration utilizing potassium permanganate.

Answer:
Write balanced equation:

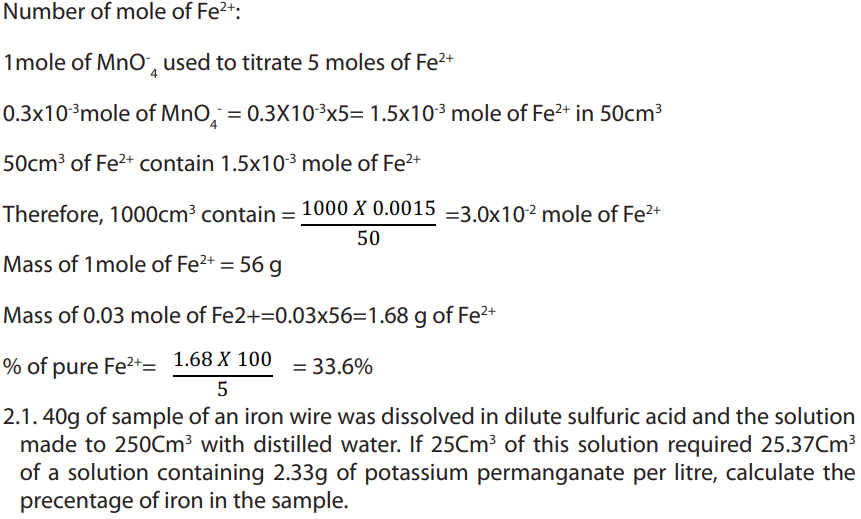
Answer
Iron dissolves in dilute sulphuric acid by the reaction equation:
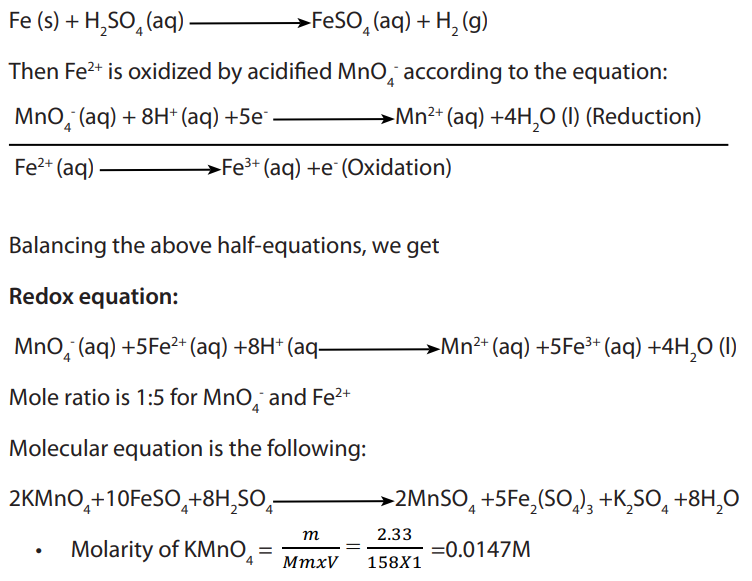


11.5.2. Titration of oxalic acid or oxalates by potassium manganate (VII)
Answer
The reducing agent is the oxalate ion,
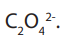 Oxalic acid is a white crystalline
Oxalic acid is a white crystalline solid of formula
The reaction between the oxalate ion and manganate (VII) ion is kinetically slow at
temperature below Titrations at room temperature take too long time for the
Titrations at room temperature take too long time for the
purple color of the manganate (VII) ion to disappear. In the oxalate ion, carbon has the
oxidation state of +3; when reacted with potassium permanganate the oxidation state
changes into +4. Titrations involving the oxalates are a little more involved, but the
results were very good. The reaction below describes the overall redox reaction:
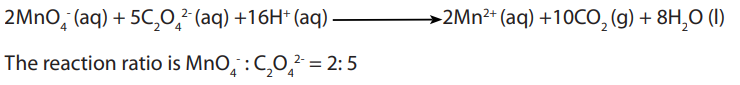

Answer:Overall equation is:
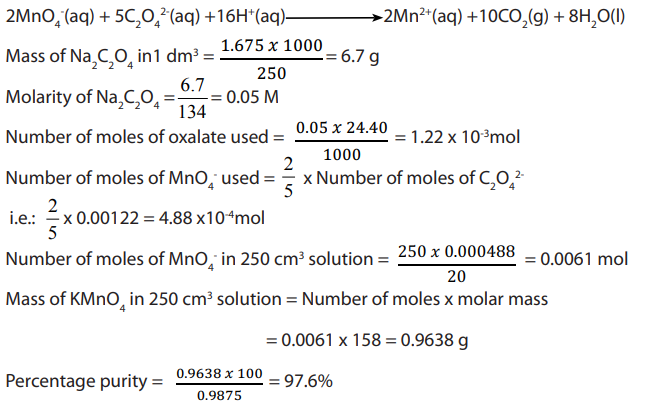
Procedure
i. A fixed mass 2.4 g of calcite is weighed and dissolved in dilute hydrochloric acid
ii. To this solution is then added ammonia solution until the solution is alkaline
and this is followed by addition of ammonium ethanedioate (oxalate) toprecipitate all calcium ions as calcium oxalate
iii. The precipitate is then filtered off, washed and then dissolved in a minimum
dilute sulphuric acid solution and then solution made up to in a
in a
volumetric flask with distilled water.
iv. of the resultant solution in (iii) is pipetted and to it is added 20 cm3
of the resultant solution in (iii) is pipetted and to it is added 20 cm3
of diluted sulphuric acid and the mixture is heated to about 70 °C.v. The hot solution is then titrated with 0.02M potassium permanganate solution
Results: of the oxalate solution required of 0.02M
of the oxalate solution required of 0.02M 

solution for complete reaction which is detected by the intense purple colour.
a. Write down the overall equation for the above sequence of analysisb. Calculate the percentage of purity of calcite
Answer:

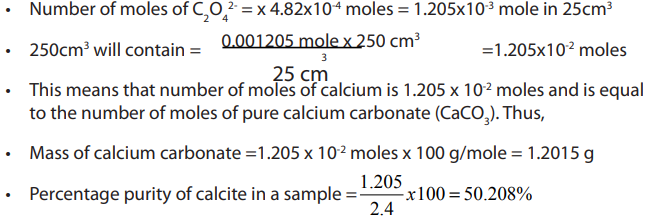
Checking up 11.5.2

11.5.3. Titration of hydrogen peroxide by potassium manganate (VII),

Activity 11.5.3
Hydrogen peroxide is a powerful reducing agent in the presence of a strong oxidizing
agent such as potassium permanganate. For this reason dilute concentrations of both
compounds must be used. A common laboratory experimentally determines the
concentration of commercially sold hydrogen peroxide (about 3%) via titration with
potassium permanganate (usually prepared to be approximately 0.02 M). Concentratedsolutions of these reactants will react explosively and must be avoided.
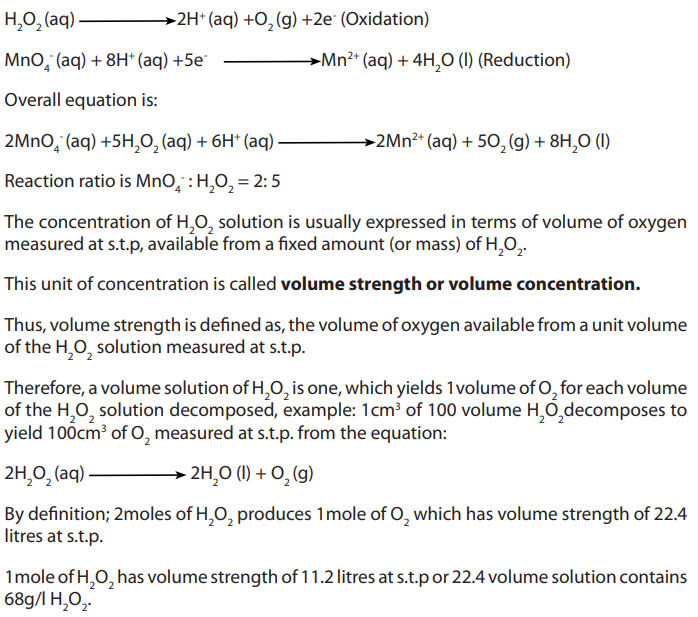
Example:


Answer
Write the balanced equation
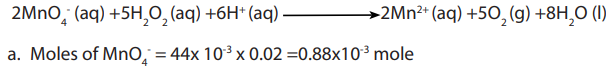
Note: The advantages of potassium permanganate as oxidizing agent are:
i. It acts as its own indicator (i.e. purple color)
ii. The crystals are obtained at high state of purity
iii. The crystals are anhydrous and not deliquescent
iv. It has a fairly high relative molecular mass
v. A wide range of substances can be oxidized by it.
However, potassium permanganate has disadvantages:
i. The crystals are not very soluble in water
ii. The compound is decomposed by the light
iii. The compound is reduced by water and organic matter from atmosphereiv. The meniscus of the solution may be difficult to see
Checking up 11.5.3

11.5.4. Titration with potassium dichromate

Activity 11.5.4
Discuss the advantages and disadvantages of using
Potassium dichromate (VI) unlike potassium manganate (VII) is a primary standard. In as an oxidizing agent in titration.
as an oxidizing agent in titration.
the titration of potassium dichromate (VI), the color change is from orange to green
and this makes it not possible to detect the sharp end-point.
Thus, an indicator is necessary for this reaction. Therefore redox indicator such as
barium diphenylamine sulphonate must be used, which changes its colour to blue atthe end point.
Potassium dichromate (VI) is a weaker oxidizing agent than potassium manganate (VII).
As such it cannot oxidize the chloride ion to chlorine, and therefore hydrochloric acid
in addition to sulphuric acid can be used to acidify solution of potassium dichromate(VI).
The reactions of potassium dichromate (VI) are similar to those of potassium manganate
(VII), however at the end-point the color is green due to the presence of chromium(III) ions that is;

a. Determination of the concentration of iodide ions.
Since iodide ions can be oxidized to molecular iodine by dichromate (VI) ion,potassium dichromate (VI) can be used as a primary standard in this reaction.
The amount of iodine liberated in the reaction is determined by titrating it against a
standard solution of sodium thiosulphate using starch indicator.
b. Analysis of iron (II) salts
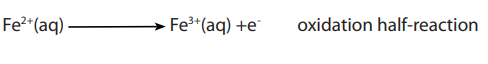
Overall equation is:

c. Analysis of nitrite salts
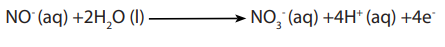
Advantages of
Unlike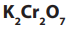 over
over 
 is used as a primary standard i.e. it can always be obtained pure;
is used as a primary standard i.e. it can always be obtained pure;
it is stable under ordinary conditions, its aqueous solution can be stored for long timeif sufficiently protected from evaporation.
Examples 2 :

Answer:
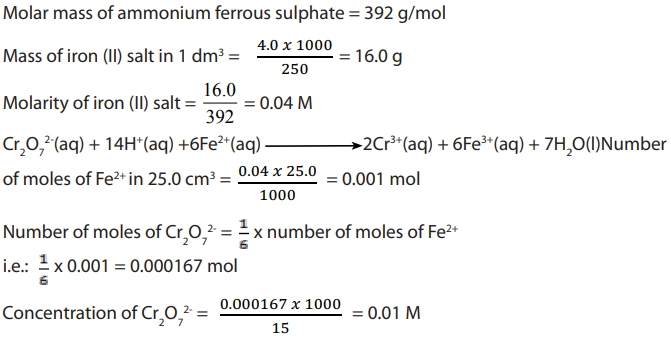
Examples 2:

Answer:
Write balanced equation
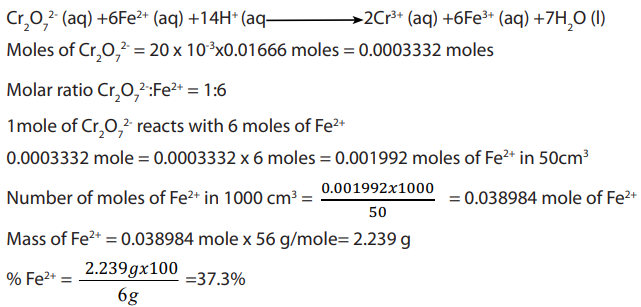
Checking up 11.5.4
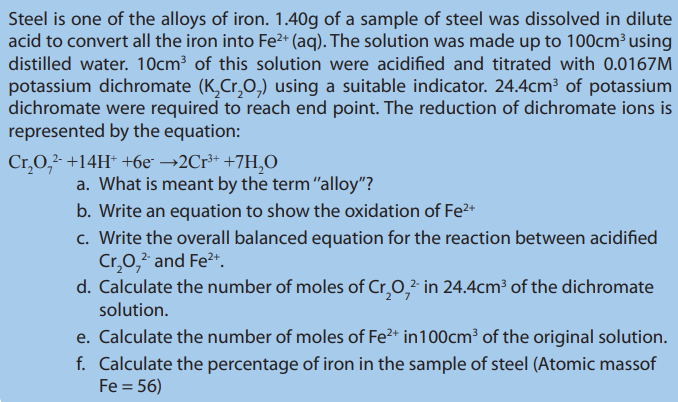
11.5.5. Titrations involving sodium thiosulphate and iodine
Activity 11.5.5
Sodium thiosulphate is a white crystalline solid with the formula
It is not a primary standard as the water content of the crystals is variable.
The solid acts as a reducing agent in a redox reaction and the reducing agent being thethiosulphate ion,

However, the thiosulphate ion is very sensitive to acid (weak or strong). In presence
of an acid (hydrogen ions), it decomposes to sulphur dioxide and elemental sulphur(which settles as a yellow precipitate).
Therefore, solutions of sodium thiosulphate should never be acidified.
The thiosulphate ions reduce iodine according to the reaction below:

Overall equation is:

a. Detection of the end point:
In titrations involving iodine and sodium thiosulphate, starch is normally used as anindicator.
Addition of the starch produces iodine-starch complex which is blue. Further drop
by drop addition of the thiosulphate solution is continued until the solution turns
colorless which marks the end point of titration.
Thiosulphate titration has important applications in the laboratory and water
treatment plants.
Potassium iodate (V), potassium dichromate (VI), iodine, potassium manganate (VII)
solutions can be used to standardize sodium thiosulphate solutions.
For example, potassium iodate (V) reacts with iodide ions in presence of an acid toliberate iodine.
The liberated iodine is then titrated against standard sodium thiosulphate using the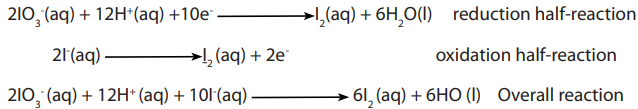
starch indicator.

b. Preparation of starch solution:
0.5g of soluble starch powder is mixed with water into a thin smooth paste which is
then poured into of boiling water. The mixture is then boiled after for about a
of boiling water. The mixture is then boiled after for about a
minute. The solution is then cooled and used when still fresh.But starch solution may be preserved by addition of few crystals of mercuric iodide.
Example 1 :

Answer:
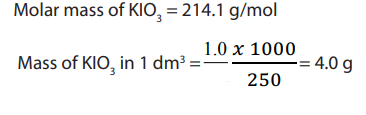
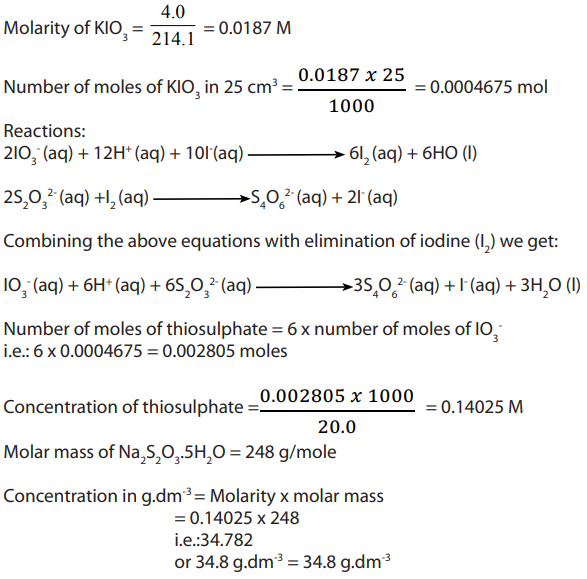
Example 2 :
A weighed sample of impure solid potassium iodate (V) of 0.80g is dissolved in
water and made up to of solution in a volumetric flask.
of solution in a volumetric flask.  of this
of this
solution is reacted with excess potassium iodide solution, to liberate iodine.
Find the percentage of the purity of potassium iodate (V). In the titration, sodium thiosulphate were needed to react with the liberated
sodium thiosulphate were needed to react with the liberated iodine.
Answer:
Write balanced equations:


c. Finding the percentage of copper in copper ore or in its salt
There are two possible methods depending on the copper ore (or salts).
a. In the first method which is applicable to any copper salt, a solution of excess
potassium iodide is added to a solution of the copper salt. A dark brown mixture
is produced (white precipitate stained brown). The brown colour is due to iodineliberated.
The iodine liberated in the reaction is now titrated with a standard solution of sodium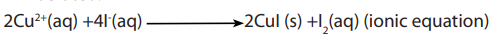
thiosulphate in the second reaction according to the following equation.
b. The second applicable method is to liberate iodine from iodate (V) ions, then titrate
it with thiosulphate ions as indicated by the following equations:
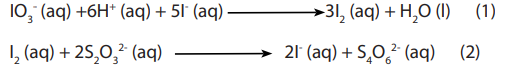
Examples:
An experiment was carried out in a laboratory to determine the percentage of copper
in sample of impure copper metal. Nitric acid was added to a sample of impure
copper metal. The resulting copper (II) nitrate was reacted with excess of potassium
iodide. The iodine liberated was titrated with a solution of sodium thiosulphate of
concentration 0.480M. The volume of sodium thiosulphate required was
Use the following equations in your calculations.
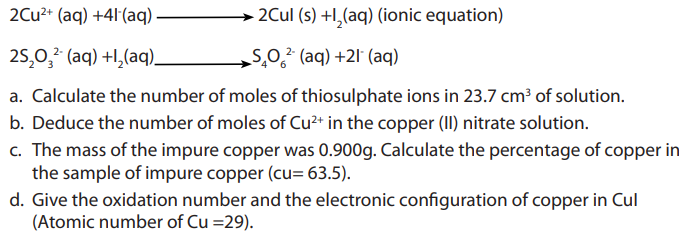
Answer:
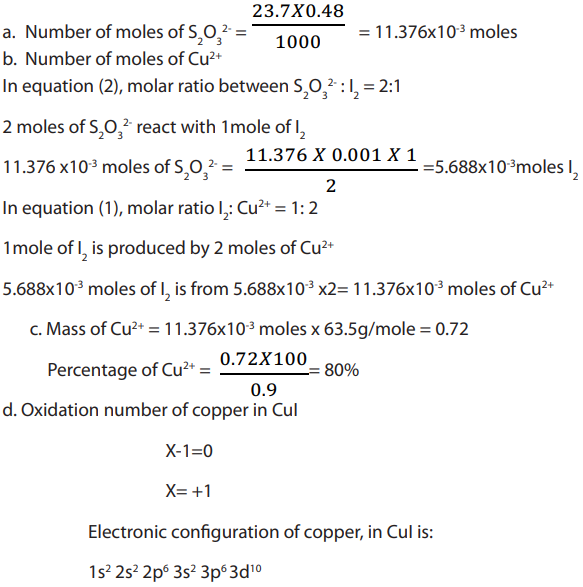
d. Determination of the percentage of available chlorine in a liquid
The method consists of measuring a fixed volume of the original liquid and then dilute
it to with distilled water. Aliquot portions of this solution is pipetted and added
with distilled water. Aliquot portions of this solution is pipetted and added to an excess of KI solution acidified with ethanoic acid.
The iodine liberated is then titrated, for example, with a standard solution of sodium
thiosulphate. This is a displacement (redox) reaction in which, aqueous potassium
iodide reacts with chlorine to form potassium chloride and iodine. The equation forthe reaction is:

Examples:
1. Concentration of chlorine in treated water for domestic use can be monitored by
testing water samples. In one such test, excess potassium iodide (KI) was added to
 sample of water.
sample of water.
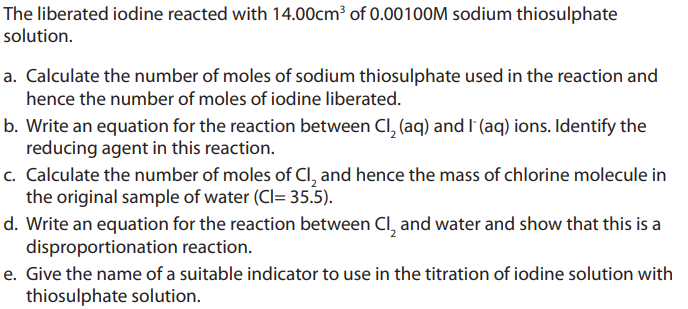
Answer:
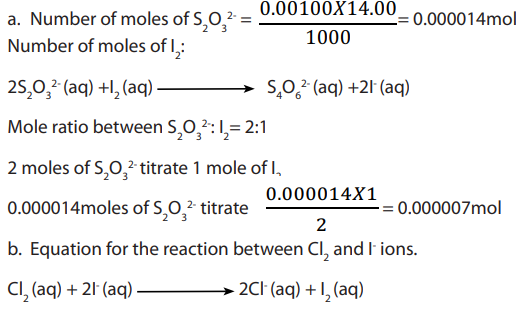
Reducing agent is I-(iodide ions)
c. Equations:
In the above reaction, chlorine underwent both oxidation and reduction, hence it is a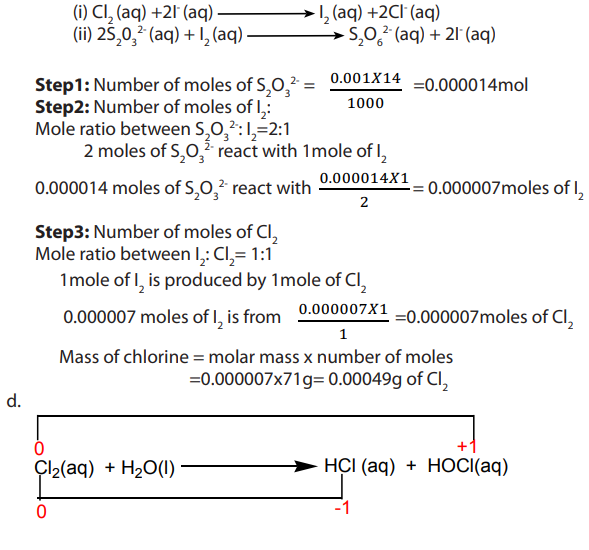
disproportionation reaction.
e. Starch solution, which changes colour from white to blue black.
Determination of the percentage of available chlorine in bleaching powder.
Procedure:
Solution was made by weighing 2.5g of bleaching powder. This is stirred in a little
was made by weighing 2.5g of bleaching powder. This is stirred in a little
water and the supernatant milk suspension poured into volumetric flask.
volumetric flask.
The solid residue is then mixed again with little water and the fine suspension decanted
into the volumetric flask.
This process is continued until most of the sample has been transferred.The solution is then made up to
The flask is then shaken vigorously and immediately mark.
mark. 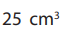
of the suspension in pipetted into a clean conical flask.
of 2M sulphulic acid or 2M ethanoic acid is added followed by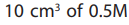
of 0.5M potassium iodide. The liberated iodine is then titrated with solution FA2which is 0.1M sodium thiosulphate solutions using starch indicator.
Specimen results:
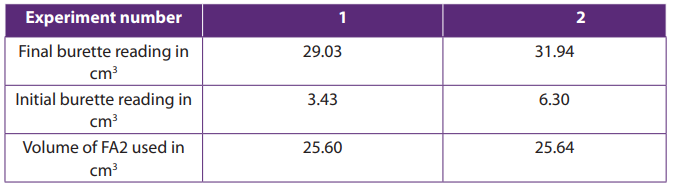
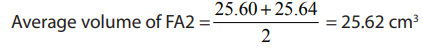
Calculations:
b. Calculate the number of moles of available chlorine in
c. Calculate the mass of the available chlorine in the sample of bleaching
powder weighed.d. Hence calculate the percentage of available chlorine bleaching powder.
Theory:
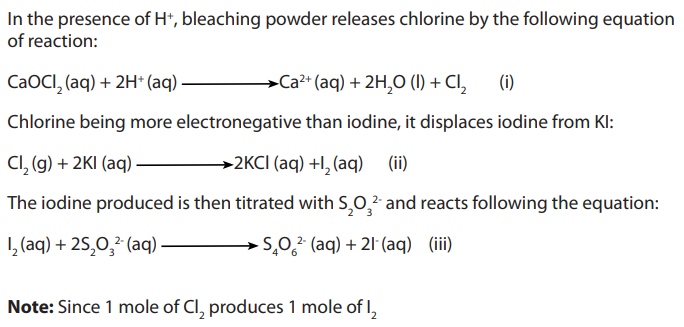
Number of moles of iodine is equal to the amount of chlorine set free.
Answers:
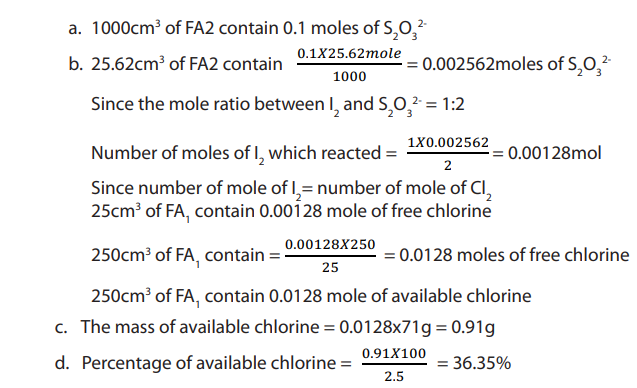
Checking up 11.5.5


11.6. Back titration
Activity 11.6
Do research and explain the term back titration and discuss the applications of backtitration.
This is a technique of determining the concentration of an unknown substance by
calculating backwards. In this case a known amount of a titratable reagent (substance)
X in excess amount is reacted with an unknown amount of substance Y to give products
and at the end of the reaction, the excess of X is titrated with a standard solution ofsubstance Z and this allows to determine the amount of Y that has reacted.
Applications of back titration:
1. Analysis of calcium carbonate materials (Examples: limestone, marble……..)
2. Determination of percentage of ammonia in an ammonium salt.3. Determination of concentration of chromates and iodides in redox titration.
Example 1 :

Answer:
Step 1: Write balanced equations

Step 2: Calculate initial number of moles of HCl

Step 3: Calculate number of HCl in excess solution
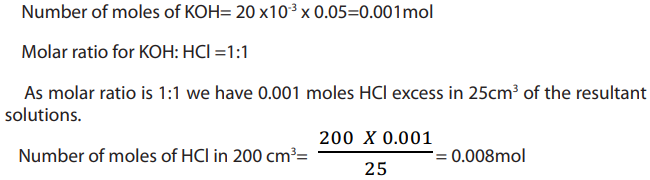
Step 4: Moles of HCl which reacts = 0.024- 0.008= 0.016mol
Initial moles of reactants = 0.024molNumber of moles in excess= 0.008mol
Step 5: Number of moles of HCl which react with


Example 2:
50cm3 of 0.45M hydrochloric acid solution were added to
of sodium hydroxide solution and the resultant solution on titration required
of 0.2M sodium hydroxide solution for complete reaction. Calculate the:
a. Number of moles of hydrochloric acid that reacted with original sodium
hydroxide solution.b. Molarity of the original sodium hydroxide solution.
Answer:

Total number of moles of HCl:
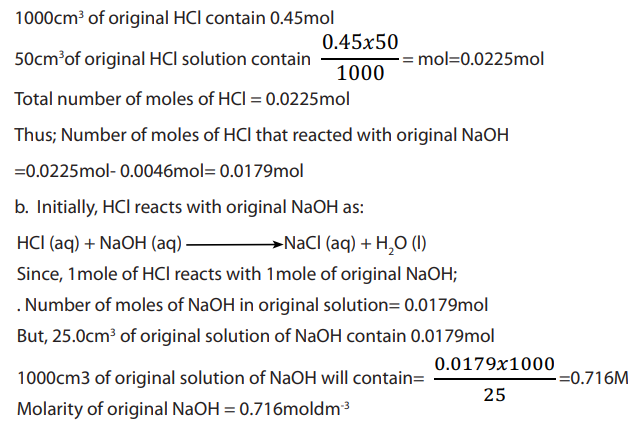
Example 3:

Answer:
a. Equation:

b.
 of the resultant solution contain 0.00478mole of NaOH
of the resultant solution contain 0.00478mole of NaOH 
c. NaOH reacts with
 Cl as shown below:
Cl as shown below:
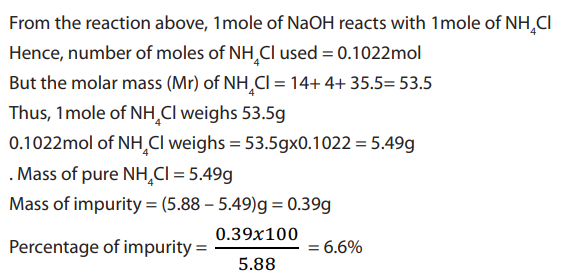
d. From the reaction in (c) above

Checking up 11.6

11.7. Applications of titration
11.7.1. Determination of the number of moles of water of crystallization
Activity 11.7.1
Do research and explain the term water of crystallization
Water of crystallization is defined as that definite amount of water
which a substance associate with on crystallizing out of an aqueous solution.
Many crystals cannot form without the presence of water i.e. water of hydration.
Examples of some hydrated substances
a. Weighing method
This method is preferably used when the crystals are neutral, and they cannot reactwith acid or basic solution.
Procedure:
• Weigh the mass of a clean dry crucible with lid.
• Two or three grams of hydrated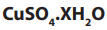 are added and the whole set up is
are added and the whole set up is
weighed.
• The crucible, lid and the content are heated on a pipe-clay triangle on a tripod
stand, gently at first and later strongly, to drive off water of crystallization.
• The set up is allowed to cool in a dessicator (to exclude moisture) and
reweighed.
• Heat again the crucible, lid and content and then cool as before and weigh
again.
• Repeat the process of heating, cooling and reweighing until a constant mass is
reached which shows that all water has been expelled.
• Using calculations, it is possible to get x moles of water of crystallization foundin one mole of substance. See the following example:
Example:
4.99g of hydrated copper (II) sulphate,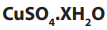 was strongly heated until
was strongly heated until
all water of crystallization was eliminated. The mass of anhydrous copper
sulphate was found to be 3.19g. Calculate the number of moles of water of
crystallization contained in one mole of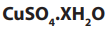 (Cu = 63.5, S = 32, O
(Cu = 63.5, S = 32, O =16, H = 1)
Answer:
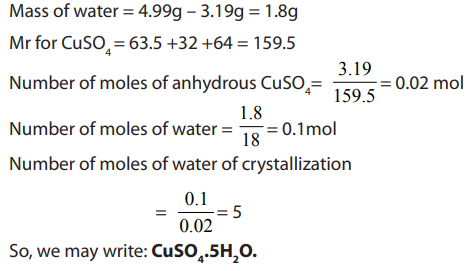
b. Using titration

Answer
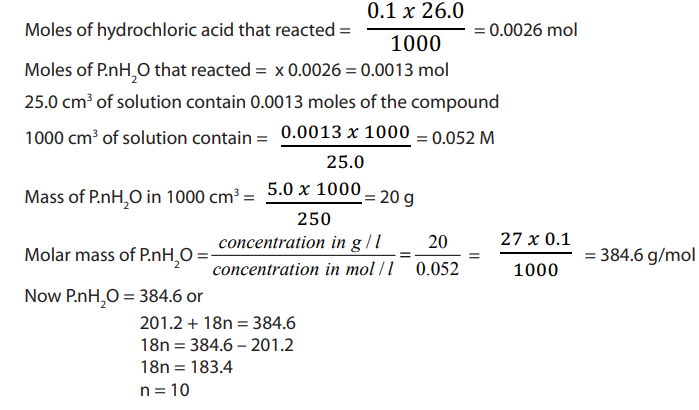
Checking up 11.7.1

11.7.2. Determination of atomic masses
Activity 11.7.2
4.99g of hydrated copper (II) sulphate, was strongly heated until
was strongly heated until
all water of crystallization was eliminated. The mass of anhydrous copper
sulphate was found to be 3.19g. Determine the number of moles of water ofcrystallization(x).
It is possible to find the atomic mass of the metal atom in titrating a basic compoundwhich reacts with acidic solution.
Example:

Answer:
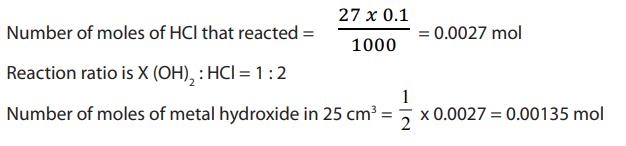

Checking up 11.7.2

11.8. End Unit Assessment
1. Explain the following terms:
a. Standard solutionb. Primary standard.
2. Describe the characteristics of a primary standard.

a. Calculate the value of x in the formula
b. Calculate the value of y in the formula
c. Calculate the value of z in the formula
d. Calculate the molecular mass of the oxalatee. Calculate the number of water of crystallization n.
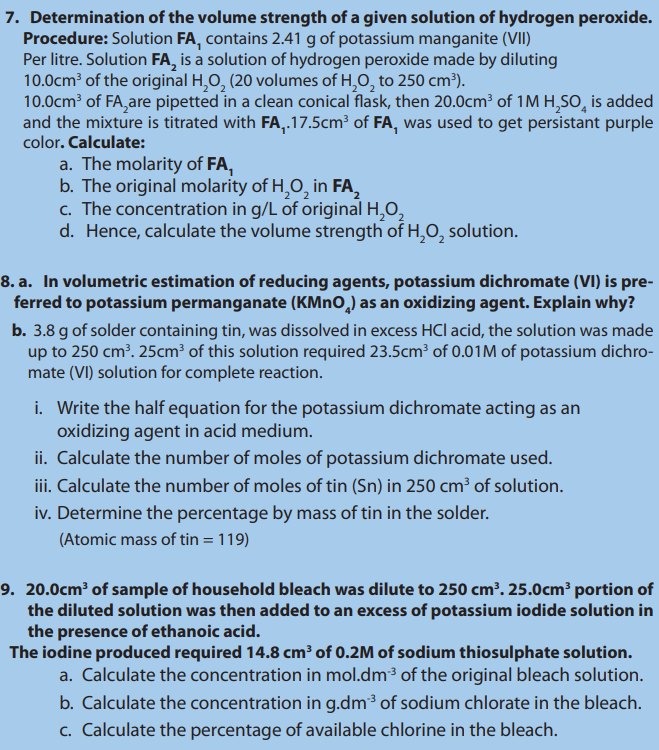

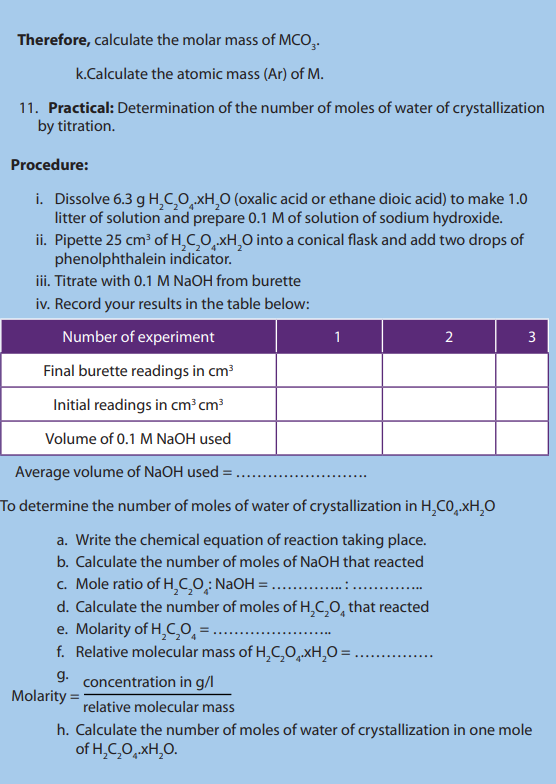

UNIT 12: CONDUCTIVITY OF SOLUTIONS
Key unity competence:
To be able to: Explain the effect of different factors on the molar conductivity ofdifferent electrolytes and the applications of conductivity measurements.
Learning objectives:
• Explain the conductivity of solutions.
• State and explain the factors that affect molar conductivity of solutions.
• State Kohlrausch’s law of individual molar conductivity.
• Use Kohlrausch’s law to calculate the molar conductivity of an electrolyte.
• Interpret a graph of molar conductivity against concentration for both weak
and strong electrolytes.
• Compare and contrast metallic conductivity and electrolytic conductivity.
• Develop a team approach and responsibility in performing experiments.
• Appreciate the contributions of other scientists like Kohlrausch’s law in
calculation of molar conductivity of solutions.• Respect the procedure in performing experiment.
12.1. Conductance of electrolytic solutions
Introductory activity

The above set up is made by
1. Solution of sodium hydroxide
2. Oranges containing citric acid
3. Solution of sugar
Carry out the three experiments as illustrated on the picture and answer to therelated questions.
1. compare the intensities of lights in set up 1,2 and 3
2. Why there is no light in set up three ?
3. What do you think are the main cause of bulbs light in set up 2 ?4. Why the light in the setup 1 and 2 are different?
Activity 12.1:
You have certainly heard about people being accidently electrocuted whenbathing at home; can you explain?
The conductance of material or solution is the property of materials due to which
a material allows the flow of ions or electrons through itself and thus conducts
electricity. It is generally defined as the reciprocal of resistance of that material. SI
unit of conductance is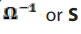 (Siemens), named after the 19th century German
(Siemens), named after the 19th century German
engineer and industrialist Ernst Werner von Siemens. It used to be called the mho
which is just ohm written backwards because the resistance is expressed in
(Ohm). The symbol for conductance is L or G. Thus G = 1/R. R = V/I soconductance is just the inverse of R: G = I/V.
The conductance of a material depends on the nature of material, number of valence
electrons for a material and temperature. Metals are good conductors of electricity
due to number and the mobility of their valence electrons. We observe that theconductance of materials decreases with increase the temperature.
Water in its pure state is known to be nonconductor because there are very little ions
The presence of electrolytes further enhances the conductivity as they supply their ions
to the solution. The conductance of electricity by ions present in the solutions is calledelectrolytic or ionic conductance.
The conductance is the inverse of resistance, therefore it is determined by calculatingthe resistance of the electrolytic solution or using the conductance cell (Figure 12.1)
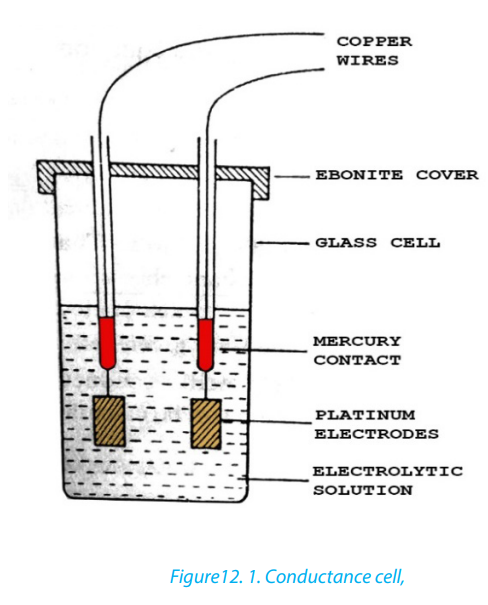
Source B.S, BAHL essentials of physical chemistry, page 476
Equivalent conductance is again called conductivity (Λ) which is the ability of a
solution to conduct electric charges, it is measured in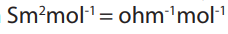
(Table 12.1)
Table 12.1: Electrochemical properties, their symbols and units
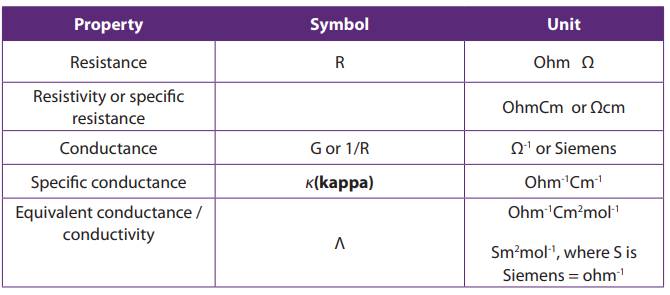
Source: B.S BAHL(2000),essentials of physical chemistry, Page 697
Checking up 12.1
a. Define conductivity
b. What is the difference between conductance and resistance?
Conductivity: Definition and description
Conductivity of a substance is defined as the ability or power to conduct or transmit
heat, electricity, or sound›. Its units are Siemens per meter [S/m] in SI and milliohmsper centimeter [m mho/cm] in U.S. customary units.
12.2. Measurement of conductivity of solutions
Activity 12.2
1. Refer to daily activity usage in electricity domain what are the objects
used to measure the voltage?
2. Refer to introductory activity above, how will you know that a solutionis conducting or not?
The conductivity is the reciprocal of the resistance (1/R) and is measured in Siemensor mhos.
Conductivity measurements are used routinely in many industrial
and environmental applications as a fast, inexpensive and reliable way of measuring
the ionic content in a solution. For example, the measurement of conductivity
is a typical way to monitor and continuously trend the performance of waterpurification systems.
Electrical conductivity meter
Principle of the measurement
The electrical conductivity of a solution of an electrolyte is measured by determining
the resistance of the solution between two flat or cylindrical electrodes separated
by a fixed distance. An alternating voltage is used in order to avoid electrolysis. The
resistance is measured by a conductivity meter. Typical frequencies used are in the
range 1–3kHz. The dependence on the frequency is usually small, but may become
appreciable at very high frequencies.
A wide variety of instrumentation is commercially available. There are two types of
cell, the classical type with flat or cylindrical electrodes and a second type based
on induction. Many commercial systems offer automatic temperature correction.
Tables of reference conductivities are available for many common solutions.
The conductivity of an electrolyte is the conductance of a volume of solution
containing one mole of dissolved electrolyte placed between two parallel
electrodes 1dm apart and large enough to contain between them all the solution;the conductivity is affected by temperature.
Checking up 12.2
Describe the functioning of conductivity meter and derive the formula ofcalculation of conductivity.
12.3. Specific conductivity of solutions
Activity 12.3:
1. Define resistivity
2. Establish a relation between conductivity and resistivity and among the
following substances, which ones areconductors and non-conductors,
for each you have to explain why they are or not conductors: pure
water, sugar, iron plate, clothes, plastic bags, ammonia solution, saltsolution, etc…
Specific Conductivity (better known as specific conductance) is the measure of
the ability of that material to conduct electricity. It is represented by the symbol “К”.
Hence, by definition, the specific conductance (specific conductivity), κ (kappa) is
the reciprocal of the specific resistance. The SI unit of conductivity is Siemens permeter (S/m).
Specific conductivity or conductivity of an electrolytic solution at any given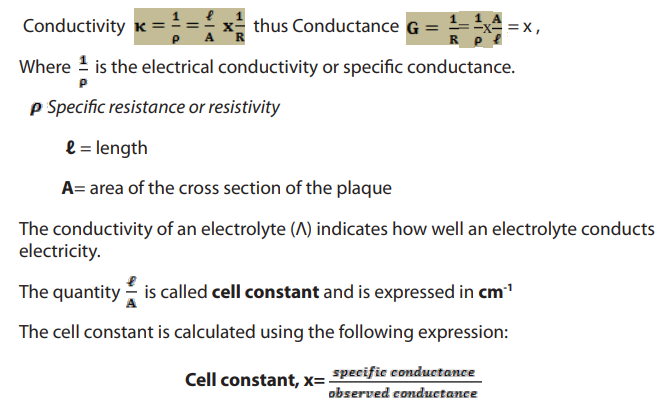
concentration is the conductance of one unit volume of solution kept between
two platinum electrodes with the unit area of cross section and at a distanceof unit length. What is the difference between Conductance and Conductivity?
. Conductance depends on the dimensions of the conductor, but conductivity does
not depend on the dimensions.. Conductance is measured in Siemens while conductivity is measured in Siemens per meter.
Checking up 12.3
12.4. Molar conductivity of solutions
Activity 12.4:
Refer to experiment done in the activity one (introductory activity) repeat the
same experiment at different concentration 1M of NaCl and 2M of NaCl, explainhow the intensity of light change with concentration
The molar conductivity of a solution at any given concentration is the
conductance of the volume of solution containing one mole of electrolyte kept
between two electrodes with the unit area of cross section and distance of unit
length. In general terms, it is defined as the ratio of specific conductivity and theconcentration of the electrolyte.
Molar conductivity of electrolytes increases with dilution. Friedrich Kohlrausch,
in 1900, experimentally found that: are the molar conductivity at a given concentration and at infinite dilution
are the molar conductivity at a given concentration and at infinite dilution
respectively, b is a constant depending on the viscosity of the solvent
and c is the concentration.Example:
Table 12.1: Variation of conductivity in terms of concentration at different temperatures
This table shows that the conductivity increases with increasing concentration and
temperature.
12.4.1. Strong electrolytes
For strong electrolyte, molar conductivity increases steadily with dilution until it
reaches the maximum value at infinite dilution (at high concentration, the lower
conductivity values are due to ionic interference. The formation of ionic pairs or
triplet and symmetrical spheres greatly reduces the mobility of ions however as
the dilution increases, there is reduced ionic interference as result of many solvent
molecules surrounding the oppositely charged ions thus an increase in molar
conductivity.
At infinite, there is independent migration of ions that is ions experience negligible
ionic interference and move independent of each other.
The molar conductivities of strong electrolytes are high. This is because, by nature,
strong electrolytes are highly dissociated when molten or when in solution into
large number of ions. These ions are mobile, hence they migrate to the electrodes,
resulting in the high conduction of electricity: the higher the number of ions arefree in solution, the higher the conductivity.
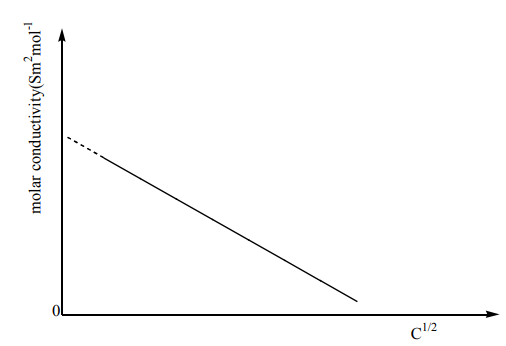
This graph can be obtained by extrapolation of the graph to zero concentration.
12.4.2. Weak electrolytes
Weak electrolytes show partial dissociation in solution, producing few ions, which
results in low conduction of electricity.
A weak electrolyte dissociates to a much lesser extent so its conductance is lowerthan that of a strong electrolyte at the same concentration.

The very large increase at infinite dilution is because the ionization increases and so
the number of ions in solution increases. The value of cannot be obtained
cannot be obtained
by extrapolation as can be seen on the graph. It is obtained by applying Kohlrausch’slaw (see later).
Summary:
• The higher the number of ions per unit volume in solution, the greater the
conductivity of the electrolytic solution. This means that the conductivity
increases with concentration of ions in solution up to an optimum level over
which it starts decreasing.
• On the other hand when the conductivity has decreased due to very high
concentration of ions, it can be increased with dilution (i.e. lower concentrations)
up to its optimum, beyond which further dilution will decrease conductivity.
• The decrease or increase of conductivity by concentrating or diluting the
solution is sharp in strong electrolytes while it is gradual in weak electrolytes.
The following graph shows.

• Λ values for strong electrolytes are larger than weak electrolytes for the same
concentration.
• Increase Λ for strong electrolyte is quite small as compared to that for weak
electrolyte towards dilution.
The table below shows the trend in conductivity with dilution for a strong and a
weak acid.
Table 12.2. Trends in conductivity

Explanation of Increase in Conductivity with Dilution:
With increase in dilution (decrease in concentration), ions become farther apart, and
inter-ionic forces (i.e. forces of attraction between unlike ions and forces of repulsion
between like ions) decrease considerably, so that greater number of ions are able to
migrate to the electrodes. In addition, due to change in equilibrium, the electrolyte
undergoes further ionization from the same mass in solution (in order to balancethe effect). Hence, more ions (conducting species) are introduced into the solution
12.5. Molar conductivity at infinite dilution
Kohlrausch’s law of independent migration of ions states that “at infinite dilution,
where ionization of all electrolytes is complete and where all interionic effects
are absent, the molar conductivity of an electrolyte is the sum of the molarconductivities of its constituent ions at constant temperature”
According to the law, the molar conductivity of KCl at infinite dilution
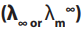 is
presented as:
is
presented as:

Some values of conductivity at infinite dilution
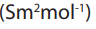
Example 1
The molar conductivity of chloride ion is What is the
What is the
molar conductivity of sodium ions given that the molar conductivity of NaCl is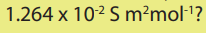
Answer:
According to Kohlrausch’s law,

Examples 2

Answer
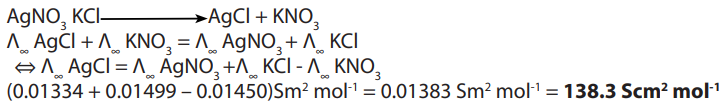
Example 3calculate the molar conductance of aqueous
 solution at infinite dilution
solution at infinite dilutionGiven
Checking up 12.4

12.6. Factors that affect molar conductivity of solutions
Activity 12.5.
Compare the conductivities of the following solutions and explain why they aredifferent

1. Temperature
The increase of temperature decreases inter-ionic attractions and increases kinetic
energy of ions and their speed. Thus, increases with temperature.
increases with temperature.
2. Concentration of the solution
The concentrated solutions of strong electrolytes have significant interionic
attractions which reduce the speed of ions and lower the value of The dilution
The dilution
decreases such attractions and increases the value of
3. Nature of electrolyte
The strong electrolytes like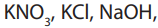 etc are completelyionized in aqueous
etc are completelyionized in aqueous
solution and have high values of molar conductivity. The weak electrolytes are ionized
to a lesser extent in aqueous solution and have lower values of molar conductivity.
Solvents of high dielectric constant yield more conducting solution. The viscosity is
inversely proportional to the conductance.
4. Ionic charge and size
Generally, the ions move at very low speeds. The velocities of hydrogen ions and
hydroxyl ions are relatively high. They contribute greatly to high conductivities ofaqueous solutions of strong acids and alkalis.
The differences in speeds of ions under similar conditions are as a result of their
difference in charge and size.
a. Ionic charge
Multiple charged ions get strongly attracted to the oppositely charged electrode.
This increases their speeds compared to singly charged ions.
b. Ionic size
Velocities of smaller ions are higher than those of larger ions of the same charge.
This is because larger ions meet many obstacles compared to small ones. However,
as ions exist in aqueous solution in a solvated form, the radius of the hydrated
ion is considerably larger than the crystal radius. Small ions get more hydrated
than larger ones due to high charge density. This reverses the expected order of
ionic velocities. Thus for group 1cations, the ionic radius increases in the order and the electric mobility increases in the same order
and the electric mobility increases in the same order  This is because of the effect of hydration.
This is because of the effect of hydration.
This explains why lithium ions have a lower molar ionic conductivity than potassium ions.
5. PressureThe molar conductance increases slightly with increase in pressure.
Checking up 12.5

Experiment
• Take two irish potatoes and wash them
• In each you have to fix the nail after you have to fix the irish potatoes on
the bench by using the glue
• Take a bulb (with two electrodes positive and negative)
• Fix also the bulb with connecting wires on the bench using also the glue
• Take the second extremity of each wire ( because the first is connected on
the bulb) and connect it on the nail fixed in the Irish potatoe• Observe the phenomenon that will happen.
12.7. Kohlrausch’s law of individual molar conductivity
Activity 12.6.
a. Given the following substances
Order those substances in their level of conductivityb. among the conductors how can you compare the conductivities

Example 1

Solution

12.7.2. Relation between molar conductivity, degree of ionization and
ionization constant
Activity 12.6.1
1. Define the following terms:
b. Degree of ionization
a. Ionization constant
2. Establish the relation of calculation of ionization constant
3. Explain why there is a relation between ionization constant and themolar conductivity
At infinite dilution, the electrolyte is completely ionized and all the ions take part
in conducting the current. At appreciable concentration, only a fraction of theelectrolyte is ionized and the degree of ionization of the electrolyte is given as

From the above equations, also the pH of the solution can be calculated.
For example, for a weak acid such as ethanoic acid,

Checking up 12.6

12.8. Use of conductivity measurement in titration and solubility product
Activity 12.7.
1. What do you understand by the term titration?
2. While titrating a solution of sodium hydroxide with hydrochloric acid,
explain how the concentration of ions change in the mixture.
3. What is solubility?
4. Define solubility product.
You have a glass of water. You add sugar or salt to dissolve. What will happen if
you continue to add sugar or salt? Can you explain?
1. What are the factors that influence solubility of a substance
2. Give an example of a substance which is insoluble or sparingly soluble
3. What is the relation between solubility of a substance and
concentration
4. Explain at which degree a sparingly soluble substance conductelectricity
12.8.1. Using conductivity to find the end point of a titration
The end-point in titration experiment can be determined using conductivity. Theprocedure of the technique is:
At the start of this titration the conical flask contains a strong alkali that
is fully ionized in water. If electrodes are placed inside the conical flask
the ions in the water will conduct electricity and a current will flow.
The more ions there are the better the conductivity and the higher the current will
be. The current can be measured using an ammeter. As acid is added to
the alkali hydrogen ions and hydroxide ions react together to form water molecules.
The number of ions in the conical flask starts to decrease and the current flowing
through the solution will decrease. At neutralization all of the hydrogen
ions and hydroxide ions have reacted together to form water molecules.
The neutral solution contains only salt ions dissolved in water molecules. The
solution will still conduct electricity because of the salt ions but the current will be
at a minimum. As more acid is added the current will start to increase because there
will now be unreacted hydrogen ions in the solution as well as the salt ions. Thesolution is now no longer neutral but has become acidic.
If you draw a graph of current against the amount of acid added you can see wherethe minimum is. This is the end point of the titration at neutralization.
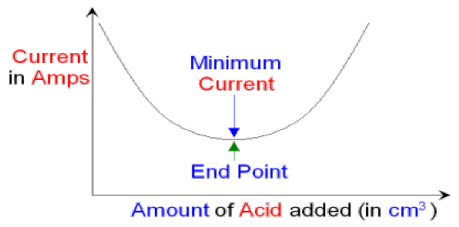
12.8.2. Determination of solubility product by conductivity measurement.
Solubility product, Ksp, is the mathematical product of its dissolved ion
concentrations raised to the power of their stoichiometric coefficients. Solubility
products are relevant when a sparingly soluble ionic compound releases ions
into solution. That is the product of the concentration of ions in the solution which
are in equilibrium with the solid ion. These concentrations can be determined viaconductivity measurements, consider the following examples :
The measurement of conductivity will depend on the value of Ksp for the sparingly
soluble substances.
The measurement of the specific conductivity, K of the saturated solution leads toa value of the concentration.


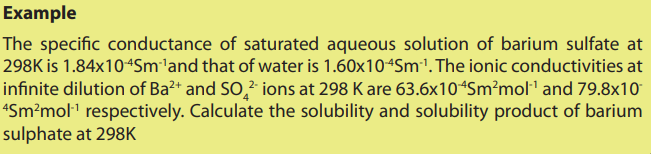

12.9. Difference between metallic conductivity and electrolytic conductivity
Activity. 12.8
Make an experiment by connecting a bulb to the batteries by using an electric
wire .After you have attempted that experience, compare the results seen
and the results you’re the introductory activity and answer to the following
questions.
a. What do you think are conductors of electricity in the two experiments
(separately)
b. Compare the reaction after 20 minutes, what is the difference betweenthe intensity of lights in the two experiments
The substances, which allow the passage of electric current, are called conductors.
The best metal conductors are such as copper, silver, tin, etc. On the other hand,
the substances, which do not allow the passage of electric current through them,
are called non-conductors or insulators. Some common examples of insulators are
rubber, wood, wax, etc.
The conductors are broadly classified into two types, Metallic and electrolyticconductors.
The electrolyte may, therefore, be defined as the substance whose aqueous solution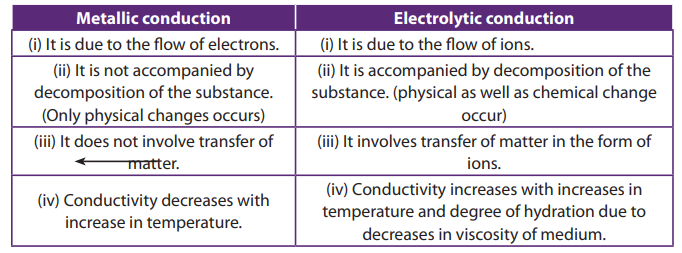
or fused state conducts electricity accompanied by chemical decomposition. The
conduction of current through electrolyte is due to the movement of ions. On the
contrary, substances, which in the form of their solutions or in their molten state donot conduct electricity, are called non-electrolytes.
Checking up 12.9
In the experiment, a student was investigating the intensity of light
In the beaker A where there was HCl solution the intensity of light was high
In beaker B where there was ethanol there was no light.
Using the plastic bag the was no light but using the copper wires there was the
intensity of light. Explain why the change in intensities of light in the aboveexperiment.




UNIT 13 : ELECTROLYSIS
Key unit competence
Predict the products of given electrolytes during
electrolysis and work out quantitatively to determine how much is liberated at agiven electrode using Faraday’s law.
Learning Objectives:
• Define electrolysis, cathode and anode.
• Explain the electrolysis of different substances.
• State Faraday’s laws and define the Faraday’s constant.
• Develop practical experimental skills related to electrolysis, interpret results,
and draw valid conclusions.
• Carry out a practical activity to explain the phenomenon of electrolysis.
• Compare the electrolysis of dilute solutions and concentrated solutions.
• Calculate the masses and volumes of substances liberated during electrolysis.
• Relate the nature of electrode, reactivity of metal ion in solution to the
products of electrolysis.• Perform electroplating of graphite by copper metal
Introductory activity
Observe carefully the figure below and answer the following question. Record youranswer and discuss them.
1. Label the set up and give the name of this Experiment.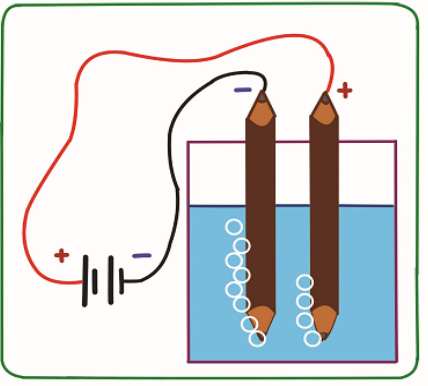
2. Suggest how water can be decomposed into hydrogen and oxygen.
13.1. Definition of electrolysis and Description of electrolyticcells.
Activity 13.1:
A.
1. In one case, you have a source of water at the top of a hill and you want
to supply water to a community in the valley down the hill
2. In another case, you have a community at the top of a hill and you
want to supply water to the community from a source located in the valley
down. Students in groups discuss how they would proceed to supply water
to the communities in the above two cases.
B. Why do we cook food by heating?
C. What is the difference between a spontaneous reaction and a non
spontaneous reaction?D. Have you heard about electrolysis? If yes, can you say what it is about?
1.Definition of electrolysis.
A spontaneous reaction is a reaction that favors the formation of products without
external energy. It is a process that will occur on its own. For example, a ball will
roll down an incline, water will flow downhill, radioisotopes will decay, and iron will
rust. No intervention is required because these processes are thermodynamically
favorable.
A non spontaneous reaction (also called an unfavorable reaction) is a chemical
reaction that necessitates external energy to occur. For example, without an
external energy source, water will remain water forever. Under the right conditions,
using electricity (direct current) will help to produce hydrogen gas and oxygen gas
from water. Cooking foods is not spontaneous reaction that is why heat is used.
Electrolyte: Sodium chloride is an ionic compound in which ions arrange themselves
in a rigid cubic lattice when in solid state. In this state, it cannot allow electric current
to pass through it. However, when it is melted, or dissolved in water, the rigid lattice
is broken, ions are free to move and electric current can pass. Therefore, it is classified
as an electrolyte.
Substances which cannot allow the flow of electric current when in molten or
in solution are referred to as non-electrolytes. When electric current (direct
current) flows through an electrolyte, it decomposes it. This phenomenon is calledelectrolysis.
Thus electrolysis is the decomposition of an electrolyte by passage of an electric
current through it. Therefore for electrolysis to take place, there must be a source
of direct current. The direct current is conveyed from its source to the electrolyte
by means of a metallic conductor and electrodes. The electrode connected to the
positive terminal of the direct current is called the anode and the one connected to
the negative terminal is the cathode. By convention, the electric current enters theelectrolyte by the anode and leaves by the cathode.
When the current passes through an electrolytic solution, ions migrate and electrons
are gained or lost by ions on the electrodes surface. Electrode that is positively
charged has deficit of electrons is called anode and the other electrode negatively
charged has excess of electrons and is called cathode. Chemical changes at theelectrodes due to the passage of electric current are called electrolysis.
2. Description of electrolytic cells.
An electrolytic cell is an electrochemical cell that drives a non-spontaneous redox
reaction through the application of external electrical energy. They are often used to
decompose chemical compounds, in a process called electrolysis . The Greek wordlysis means to break up.
Important examples of electrolysis are the decomposition of water into hydrogen
and oxygen, production of sodium metal, Na, from molten NaCl, production of
aluminium and other chemicals. Electroplating (e.g. of copper, silver, nickel or
chromium) is done using an electrolytic cell.
An electrolytic cell has three component parts: an electrolyte and two electrodes
(a cathode and an anode). The electrolyte is usually a solution of water or other
solvents in which ions are dissolved. Molten salts such as sodium chloride are also
electrolytes. When driven by an external voltage applied to the electrodes, the ions
in the electrolyte are attracted to an electrode with the opposite charge, where
charge-transferring (also called faradaic or redox) reactions can take place. Only
with an external electrical potential (i.e. voltage) of correct polarity and sufficient
magnitude can an electrolytic cell decompose a normally stable, or inert chemical
compound in the solution. The electrical energy provided can produce a chemicalreaction which would not occur spontaneously otherwise.
The main components required to achieve electrolysis are:
• An electrolyte is substance containing free ions which are the carriers of
electric current in the electrolyte. If the ions are not mobile, as in a solid salt
then electrolysis cannot occur.
• A direct current (DC) supply: provides the energy necessary to create or
discharge the ions in the electrolyte. Electric current is carried by electronds
in the external circuit.
• Electrolysis depends on controlling the voltage and current.
• Alternating current (AC) would not be appropriate for electrolysis. Because the
“cathode” and “anode” are constantly switching places, AC produces explosive
mixtures of hydrogen and oxygen.
• Two electrodes: an electrical conductor which provides the physical interfacebetween the electrical circuit providing the energy and the electrolyte.
The key process of electrolysis is the interchange of atoms and ions by the removal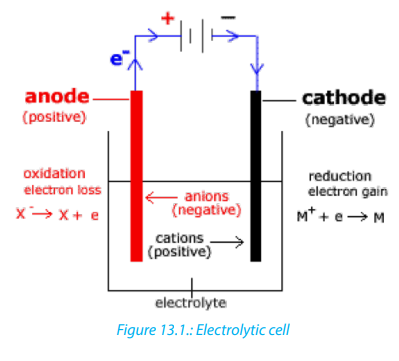
or addition of electrons from the external circuit. The products of electrolysis are in
some different physical states from the electrolyte and can be removed by some
physical processes.
Electrodes of metal, graphite and semi-conductor material are widely used. Choice
of suitable electrode depends on chemical reactivity between the electrode and
electrolyte and the cost of manufacture.
Note:
The suitable electrode in electrolysis should be inert (Cu, Pt, etc.) therefore it will not
participate in the chemical reaction.
It is very easy to be confused about the names CATHODE and ANODE and what theirproperties are, both with electrochemical cells and electrolytic cells.
(To help you to remember, Cathode is the site of reduction, or, if you prefer, CCC = CathodeCollects Cations. Anode is the site of oxidation, or, AAA = Anode Attracts Anions.)
Checking up 13.1:

13.2. Electrolysis of sodium chloride
Activity 13.2
Investigating the effect of concentration on the products formed during
electrolysis of concentrated sodium chloride solution.
Materials: Carbon or graphite rods, connecting wire, U-tube, dry cell, glasssyringes, concentrated sodium chloride, cork and switch.
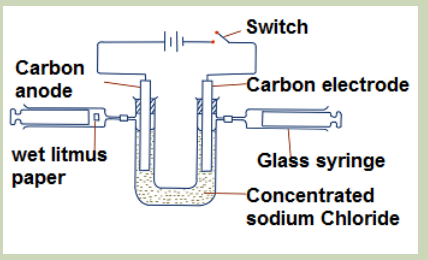
Figure 13.2.1: Electrolysis of concentrated sodium chloride solution
Procedure:
2. Add 10g of sodium chloride to 100cm3
of distilled water.
3. Warm the mixture and continue adding sodium chloride until a
saturated solution is formed.
4. Put the saturated solution in U-tube and fit it with carbon rods and glass
syringes.
5. Level the brine solution in the two arms and switch on the circuit.
Record any observations made after some time. Identify any gases
collected in the syringe.
Questions:
a. Identify the gases formed by testing them using litmus papers.
b. Using ionic equations, explain how the products are formed.
Sodium chloride may be in different forms that can be electrolytes. It may be in its
molten state, dilute solution or concentrated solution. In each case, the products of
electrolysis differ because of different factors.13.2.1. Electrolysis of molten sodium chloride
The molten salt is introduced in a container called electrolytic cell (or electrolysis
cell) in which there are two inert electrodes (platinum or graphite). Electrodes are
connected to a DC generator.
• Cations (Na+) move toward the cathode (negative electrode), where they takeelectrons and are reduced. On cathode metallic sodium is deposited:
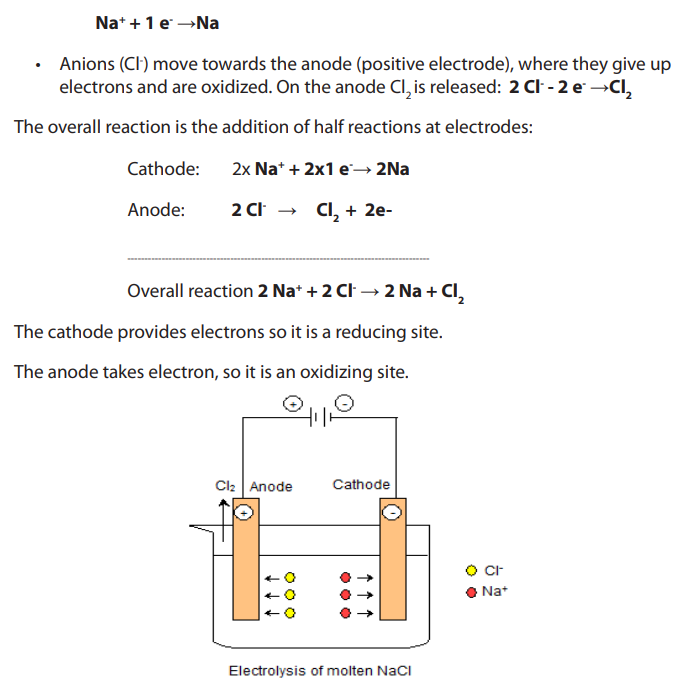
Figure 13.2.2: Electrolysis of molten sodium chloride solution
• Another important thing to note is that twice as much hydrogen is produced
as oxygen. Thus the volume of hydrogen produced is twice that of oxygen. Referto the equations above and note the number of electrons involved to help you
13.2.2. Electrolysis of Dilute Sodium Chloride Solution
An aqueous solution of sodium chloride contains four different types of ions. They
are ions from sodium chloride: Na+ (aq) and Cl-(aq)
Ions from water: H+ (aq) and OH-(aq)
When dilute sodium chloride solution is electrolysed using inert electrodes, the
Na+ and H+ ions are attracted to the cathode. The Cl- and OH- ions are attracted tothe anode.
Table 13.2: Standard reduction potentials.
The table shown below is simply a table of standard reduction potentials in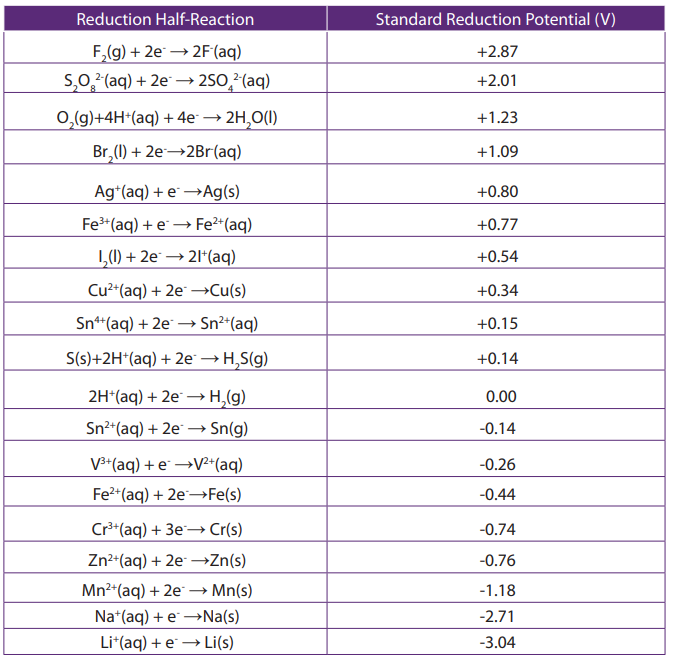
decreasing order. The species at the top have a greater likelihood of being reduced
while the ones at the bottom have a greater likelihood of being oxidized. Therefore,
when a species at the top is coupled with a species at the bottom, the one at the topwill be easily reduced while the one at the bottom will be oxidized.
• At the cathode:
The H+ and Na+ ions are attracted to the platinum cathode. H+ ions gains electrons
from the cathode to form hydrogen gas. (The hydrogen ions accept electrons more
readily than the sodium ions. As a result, H+ ions are discharged as hydrogen gas,
which bubbles off. Explanation why H+ ions are preferentially discharged will begiven later.)

• At the anode:
OH- and Cl- are attracted to the platinum anode. OH- ions give up electrons to theanode to form water and oxygen gas.
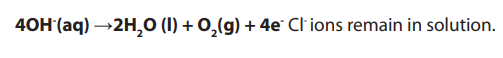
Adding the two reactions and balancing the terms:

If we remove 2 molecules of water on both sides, we get:
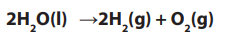
Note:
• Since water is being removed (by decomposition into hydrogen and oxygen),
the concentration of sodium chloride solution increases gradually. The overall
reaction shows that the electrolysis of dilute sodium chloride solution isequivalent to the electrolysis of water.

Figure 13.2.3: Electrolysis of dilute sodium chloride solution
13.2.3. Electrolysis of Concentrated Sodium Chloride Solution
The only difference with dilute NaCl solution is that at the anode, Cl- ions are more
numerous than OH- ions. Consequently, Cl- ions are discharged as chlorine gas, which
bubbles off.
A half-equation shows you what happens at one of the electrodes duringelectrolysis.
Sodium ions Na+ and hydroxide OH– are also present in the sodium chloride
solution. They are not discharged at the electrodes. Instead, they make sodiumhydroxide solution.
These products are reactive, so it is important to use inert (unreactive) materials forthe electrodes.
One volume of hydrogen gas is given off at the cathode and one volume of chlorine
gas is produced at the anode. The resulting solution becomes alkaline becausethere are more OH- than H+ ions left in the solution.

Checking up 13.2:
With the help of equations of reactions which occur at each
electrode, outline what happens during electrolysis of dilute aqueous sodiumchloride. What happens to the pH of the solution as electrolysis continues?
13.3. Electrolysis of water
Activity 13.3: Investigate the products formed during the electrolysis of water
Materials:
• Distilled water
• Tap water
• 2 silver-colored thumb tacks
• 9V battery
• Small, clear plastic container
• 2 test tubes
• Stopwatch
• Baking soda
• Table salt
• Lemon• Dish washing detergent

Procedure:
3. Insert the thumb tacks into the bottom of the plastic container so that
the points push up into the container. Space them so that they’re the
same distance apart as the two terminals of the 9V battery. Be careful not
to harm yourself!
4. Place the plastic container with the terminals of the battery. If the cup is
too large to balance on the battery, be sure thumb tacks are connect to
positive and negative pushpins and do no touch each other.
5. Slowly fill the container with distilled water. If the tacks move, go ahead
and use this opportunity to fix them before you proceed. Will distilled
water conduct electricity on its own? Try it!
6. Add a pinch of baking soda.
7. Hold two test tubes above each push pin to collect the gas being formed.
Record your observations. What happens? Does one tube have more
gas than the other? What gases do you think are forming?
8. Discard the solution, and repeat the procedure with a different
combination:
• Distilled water and lemon juice
• Distilled water and table salt
• Distilled water and dish detergent
• Distilled water (no additive)
• Tap water (Does tap water works? If so, why?)
Question: During the electrolysis of water, which electrolyte conductselectricity the best?
Water can be decomposed by passing an electric current through it. When this
happens, the electrons from the electric current cause an oxidation-reduction
reaction. At one electrode, called the cathode, electrons cause a reduction. At the
other electrode, called the anode, electrons leave their ions completing the circuit,
and cause an oxidation.
In order to carry out electrolysis the solution must conduct electric current.
Pure water is a very poor conductor. To make the water conduct better we can
add an electrolyte (NaCl) to the water. The electrolyte added must not be more
electrolyzable than water. Many electrolytes that we add electrolyze more easily
than water. Sulfate ions do not electrolyse as easily as water, so sulfates are often
used to enhance the conductivity of the water
Water may be electrolyzed in the apparatus shown below. Pure water is however a
very poor conductor of electricity and one has to add dilute sulphuric acid in orderto have a significant current flow.
In pure water at the negatively charged cathode, a reduction reaction takes place,
with electrons (e−) from the cathode being given to hydrogen cations to formhydrogen gas. The half reaction, balanced with acid, is:
Reduction at cathode:
At the positively charged anode, an oxidation reaction occurs, generating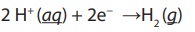
oxygen gas and giving electrons to the anode to complete the circuit:
Oxidation at anode:
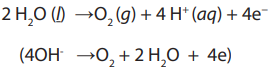
Combining either half reaction pair yields the same overall decomposition of water
into oxygen and hydrogen:
Overall reaction:
The number of hydrogen molecules produced is thus twice the number of oxygen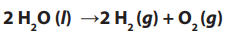
molecules. Assuming equal temperature and pressure for both gases, the producedhydrogen gas has therefore twice the volume of the produced oxygen gas.
Checking up 13.3
I understand the process of water electrolysis, that water as an electrolyte can
be decomposed into hydrogen and oxygen via an external energy source (an
electrical current). I know that the reduction of hydrogen takes place on the
cathode and the oxidation reaction takes place on the anode. I also know
that water, already partially split into H+ and OH- (though there are very few
of these ions in pure water).
1. Electric current (direct current) electrolyzes water. Discuss this
statement.2. Why alternative current are not used for the same process?
13.4. Electrolysis of concentrated copper (II) sulphate solutionusing inert electrode
Activity 13.4: Investigating what happens when a solution of copper (II)sulphate is electrolysed using carbon and copper electrodes.
Apparatus and chemicals: Glass cell, Carbon rod, 2M copper (II) sulphate
solution, connecting wires, dry cells, copper plates, propanone and litmuspaper.

Procedure:
3. Determine the mass of the graphite rods and record it.
4. Put 0.5M of copper (II) sulphate solution in a glass cell with the carbon
(graphite) rods and set up the apparatus. Carefully observe all the
changes taking place at the electrodes and the solution. Test the
resulting solution with blue litmus paper.
5. After some time, switch off the current, remove the electrodes, wash
them in propanone, dry then and then weigh them.
6. Repeat the experiment using clean strips of copper metal as electrodes.
Weigh them and then complete the circuit using freshly preparedcopper (II) sulphate solution. Record your observations.
Questions:
1. Explain the changes observed during the electrolysis of copper (II)
sulphate using :
a.Carbon electrodes
b.Copper electrodes
2. Outline the changes that occur in the solution from the beginningto the end of the experiment.
The products of electrolysing of copper sulphate solution with inert electrodes(carbon/graphite or platinum) are copper metal and oxygen gas.

Figure 13.4.1: Simple apparatus to investigate the electrolysis of aqueous solutions
Using the simple apparatus (diagram above) and inert carbon (graphite) electrodes,
you can observe the products of the electrolysis of copper sulfate solution are a
copper deposit on the negative cathode electrode and oxygen gas at the positive
anode electrode. This anode reaction differs if you use copper electrodes. You have
to fill the little test tubes with the electrolyte (dilute copper sulphate solution), hold
the liquid in with your finger and carefully invert them over the nearly full electrolysiscell. The simple apparatus (above) can be used with two inert wire electrodes.
The blue colour fades as more and more copper is deposited, depleting theconcentration of blue copper ion
Diagram for the electrolysis of copper (II) sulphate solution with carbon in solution.
in solution.electrodes.
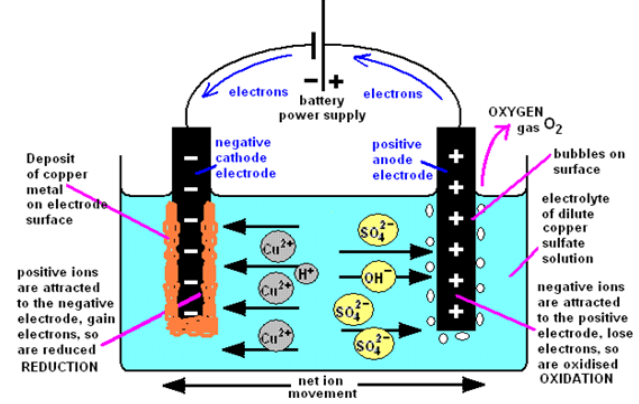
Figure 13.4.2: The electrolysis of copper (II) sulphate solution with carbon electrodes.
The electrode reactions and products of the electrolysis of the electrolyte copper
sulphate solution (with inert carbon-graphite electrodes) are illustrated by thediagram above
(a) The electrode products from the electrolysis of copper sulphate with inertgraphite (carbon) electrodes
The negative cathode electrode attracts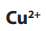 ions (from copper sulphate) and
ions (from copper sulphate) and 
ions (from water). Only the copper ion is discharged, being reduced into copper
metal. The less reactive a metal, the more readily its ion is reduced on the electrode
surface.
A copper deposit forms as the positive copper ions are attracted to the negativeelectrode (cathode)
The traces of hydrogen ions are not discharged, so you do not see any gas collected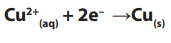 positive ion reduction by electron gain.
positive ion reduction by electron gain.
above the negative electrode. The blue colour of the copper ion will fade as the copper
ions are converted into the copper deposit on the cathode
At the positive anode reaction with graphite electrodes
Oxygen gas is formed at the positive electrode, an oxidation reaction (electronloss).
The negative sulphate ions or the traces of hydroxide ions
or the traces of hydroxide ions  are
are
attracted to the positive electrode. But the sulphate ion is too stable and nothinghappens. Instead hydroxide ions are discharged and oxidised to form oxygen.
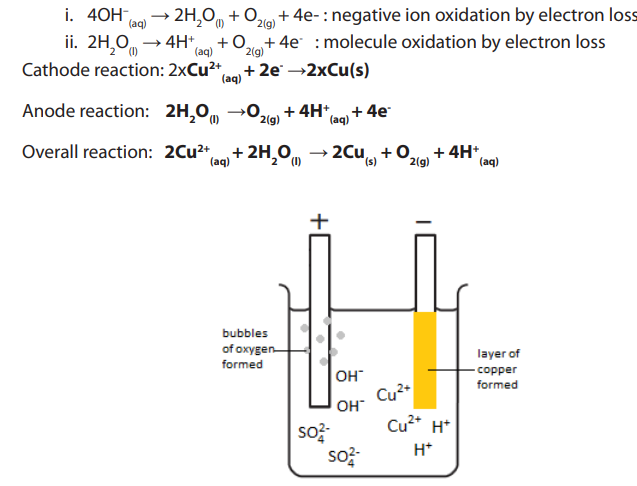
Figure 13.4.3: Electrolysis of copper(II) sulphate
Checking up 13.4
1. Name the product at the cathode and anode during electrolysis of:
a. Molten lead bromide with inert electrode.
b. Acidified copper sulphate solution with inert electrodes.
c. Acidified water with inert electrode.
d. Dilute hydrochloric acid with inert electrode.
e. Concentrated hydrochloric acid with inert electrode.
2.Predict the products formed when the following molten compounds are
electrolysed using carbon electrodes;
a. Lead(II) bromideb. Magnesium oxide
13.5. Electrolysis of concentrated copper (II) sulphate solutionusing copper electrodes
The products of electrolysis of copper sulfate solution with copper electrodes are
copper metal and copper ions (the copper anode dissolves).
The electrolysis of copper (II) sulphate solution using copper electrodes is shownbelow.

Figure 13.5.1: Electrolysis of copper (II) sulphate
Using the simple apparatus and two copper electrodes the products of the
electrolysis of copper sulphate solution are a copper deposit on the negative
cathode electrode and copper dissolves, at the positive anode electrode.
at the positive anode electrode.
This copper anode reaction differs from the one when you use an inert graphiteelectrode for the anode.
When Copper (II) sulphate is electrolysed with a copper anode electrode (thecathode can be carbon or copper), the copper deposit on the cathode (–) equals the
copper dissolves at the anode (+). Therefore the blue colour of the Cu2+ ions stays
constant because Cu deposited = Cu dissolved. Both involve a two electron transfer
so it means mass of Cu deposited = mass of Cu dissolving for the same quantity
of current flowing (flow of electrons). You can check this out by weighing the dry
electrodes before and after the electrolysis has taken place.
The experiment works with a carbon anode and you see the blackness of the graphitechange to the orange-brown colour of the copper deposit.
Figure 13.5.2: The electrolysis of copper (II) sulphate solution with a copper anode and a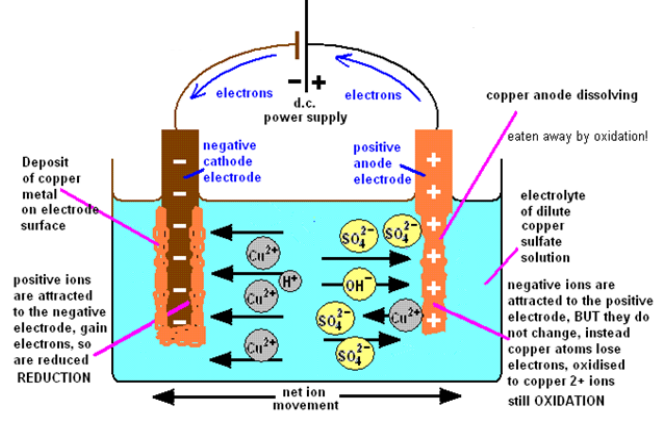
copper cathode.
The electrode reactions and products of the electrolysis of copper sulphate solution
(with a copper anode) are illustrated by the diagram above.
(a) The electrode products from the electrolysis of copper sulphate with copperelectrodes
The negative cathode electrode attracts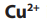 ions (from copper sulphate) and
ions (from copper sulphate) and 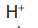
ions (from water). Only the copper ion is discharged, being reduced to copper metal.
A reduction electrode reaction at the negative cathode: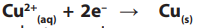
(copper deposit, reduction 2 electrons gained) reduction by electron gain.
The positive anode reaction with copper electrodes
It’s the copper anode that is the crucial difference than electrolysing copper sulphatesolution with an inert carbon (graphite) or platinum electrode.
The negative sulphate ions
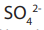 (from copper sulphate) or the traces of hydroxide
(from copper sulphate) or the traces of hydroxide
ions (from water) are attracted to the positive electrode. But both the sulphate
(from water) are attracted to the positive electrode. But both the sulphate
ion and hydroxide ion are too stable and nothing happens to them because the
copper anode is preferentially oxidised to discharge copper ions.
copper ions.
An oxidation electrode reaction at the positive anode: copper dissolves, twoelectrons are lost following the half reaction:
Copper atoms oxidised to copper (II) ions: dissolving of copper in its electrolytic
purification or electroplating (must have positive copper anode).
Copper (II) ions reduced to copper atoms: deposition of copper in its electrolytic
purification or electroplating using copper (II) sulphate solution, so the electrodecan be copper or other metal to be plated or any other conducting material.
This means for every copper atom that gets oxidised, one copper ion is reduced,therefore
when copper electrodes are used in the electrolysis of copper sulphate solution, the
mass loss of copper from the positive anode electrode should equal to the mass ofcopper gained and deposited on the negative cathode electrode.
You can show this by weighing both electrodes at the start of the experiment. After
the current has passed for some time, carefully extract the electrodes from the
solution, wash them, dry them and reweigh them. The gain in mass of the cathodeshould be the same as the loss of mass from the anode.
Checking up 13. 5
1.
a. Predict the products formed (i) at the cathode, (ii) at the anode, when
copper (II) sulphate solution is electrolysed using carbon electrodes.
b. Describe the colour changes in the electrolyte.
2. What will you observe
a. At cathode
b. At anode
c. In electrolytic, during the electrolysis of copper (II) sulphate solution
with copper electrode.
3.Write equations for the reaction taking place at cathode and at anode
during the electrolysis of:
a. Acidified copper sulphate solution with copper electrode.
b. Acidified water with inert electrode.
c. Molten lead bromide with inert electrode.
4.Using a table, highlight the similarities and differences between using
graphite electrodes and copper electrode for the electrolysis of coppersulphate.
13.6. Faraday’s Laws
Activity 13.6
Comparison of the amounts of different substances liberated by the samequantity of electricity.
Figure 13.6: Comparison of the amounts of different substances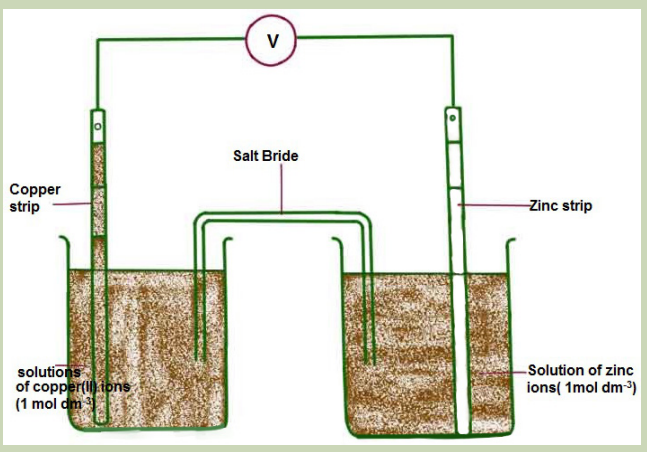
liberated by the same quantity of electricity
Set up the circuit containing a copper voltmeter and a silver voltmeter (a
voltmeter is a vessel containing two electrodes immersed in a solution of ionsthrough which a current is to be passed.)
Identify the copper and silver cathodes, clean and dry them, and after weighing
them return them in their respective voltmeters. Pass a current of about 0.5A
for 20 or 30 minutes, after which the cathodes should be removed, cleaned anddried, and reweighed.
Compare the masses of copper and silver deposited. Note that care must be
taken in removing the silver cathode from the solution as the metal does notalways adhere well to the cathode.
The relationship between the mass of product formed at an electrode and the
quantity of electricity passed through an electrolyte is given by Faraday’s laws ofelectrolysis.
Michael Faraday (1791-1867) did the first work on electrolysis and formulated what
is known today as Faraday’s laws of electrolysis.
These laws express the quantitative results of electrolysis. They assert that the
amount (expressed in moles) of an element liberated during electrolysis dependsupon:
1. The time of passing the steady current, t
2. The magnitude of the steady current passed, I3. The charge of the ions
13.6.1. Faraday’s first law
According to this law, “The amount of substance liberated at an electrode isdirectly proportional to the quantity of electricity passed”
1F = 96500 coulomb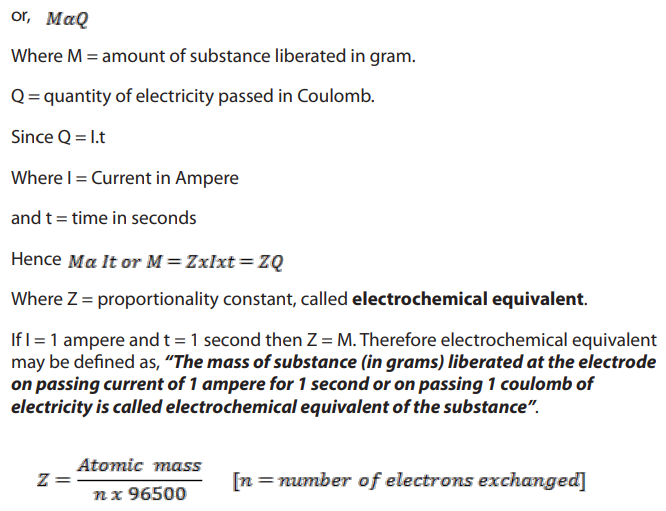
So, 1 Faraday [96500 coulomb] of electricity will produce 1 gm equivalent of Ag,
Cu and Al at cathode.
13.6.2. Faraday’s second law
According to this law, “if same quantity of electricity is passed through different
electrolytes, then the amount of substances liberated at the respective electrodesare in the ratio of their equivalent masses”.
Or
When the same quantity of electricity passes through solutions of different electrolytes,
the amounts of the substances liberated at the electrodes are directly proportional totheir chemical equivalents.
Equivalent mass is the mass of a substance especially in grams that combines with
or is chemically equivalent to eight grams of oxygen or one gram of hydrogen; theatomic or molecular Mass divided by the valence.
Example:
Calculate the amount of electric charge in coulombs which can
deposit 5.2g of aluminium when a current was passed through a solution ofaluminium sulphate for some time.
Solution:
3 moles of electrons are needed to deposit 1 mole of aluminium (24g of
aluminium).
Checking up 13.6
1. A current of 0.65A was passed through sulphate solution of metal X
for 35 minutes between platinum electrodes. If 0.449 g of the metal is
deposited at the cathode, calculate the charge on the element X (atomic
mass is 63.5)
2. When a constant current was passed through an aqueous solution
of copper (II) nitrate for one hour the mass of the copper cathode
increased by 15.24 g. Calculate the current in amperes which was used(F= 96500 Cu = 63.5)
13.7. Factors affecting Electrolysis
Activity13.7: Investigating how the nature of electrodes affects the discharge of ions during electrolysis.
Apparatus and chemicals:
• Beaker (100 cm3)
• 6V Battery
• 2 connecting wires with crocodile clips
• 2 carbon electrodes
• 2 copper electrodes
• solution
solution
Caution: Do not allow the electrodes to touch each other while the powersupply is switched on, otherwise this may damage the equipment.
A. Electrolysing of copper (II) sulphate solution using Carbon electrodes.
Figure 13.7.1: Electrolysis of copper (II) sulphate solution using carbon electrodes.
Procedure: let the current flow for 5 minutes
Observe what happens at each electrode
Questions:
1. What product is formed on the cathode?
2. What product is formed at the anode?
3. Has the colour of the solution changed?4. Explain the observations in 3.
B.Electrolysing of copper(II) sulphate solution using Copper electrodes
Procedure: let the current flow for 5 minutes
Observe what happens at each electrode
1. What product is formed on the cathode?
Questions:
2. What product is formed at the anode?
3. Has the colour of the solution changed?4. Explain the observations in 3.
In an electrolysis where there are more than one species which can be discharged
at the same electrode, only one of them is discharged at a time; for example, in an
aqueous sodium chloride solution, we have four species that is, and
and 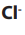
ions from sodium chloride and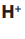 and
and  ions from water.
ions from water.
During electrolysis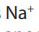 ions and
ions and 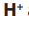 ions migrate to the cathode while
ions migrate to the cathode while  ions and
ions and
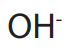 migrate to the anode.
migrate to the anode.
Now the question is, which species of ions will be discharged at the cathode and
which ones will be discharged at the anode first?The factors which decide the selective discharge of ions are:
• Nature of electrodes
• Position of the ion in electrochemical series
• Concentration• The state of the electrolyte
13.7.1. Nature of Electrodes
In the electrolysis of sodium chloride solution using a platinum cathode,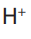 ions
ions are discharged first in aqueous solution.
However, if the cathode is mercury, sodium is discharged. The sodium atom
discharged combines with mercury cathode to form sodium amalgam.

Electrolysis of copper sulphate using copper anode
In this electrolysis, the anions, migrate to the anode but none of them
migrate to the anode but none of them is discharged. Instead the copper atoms of the anode ionise and enter the solution.
The copper (II) ions are attracted to the cathode and copper is deposited as a brown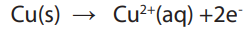
layer. The use of platinum anode gives oxygen as the product due to the reaction;
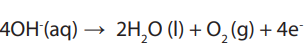
13.7.2. Position of ion in Electrochemical Series
When solving for the standard cell potential, the species oxidized and the species
reduced must be identified. This can be done using the activity series. The table 13.2
is simply a table of standard reduction potentials in decreasing order. The species
at the top have a greater likelihood of being reduced while the ones at the bottom
have a greater likelihood of being oxidized. Therefore, when a species at the top is
coupled with a species at the bottom, the one at the top will become reduced whilethe one at the bottom will become oxidized.
During electrolysis of solution containing a mixture of ions, the ion lower inelectrochemical series is discharged first in preference to the one high in the series.
Let us look at the role of water in electrolysis products.Water molecules to a small extent (degree) ionize as
Due to the above ionization, aqueous solutions of electrolytes contain two sets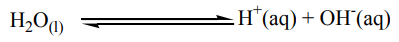
of ions that is those from the salt dissolved and the ions from water
ions from water molecules.
Example:
Electrolysis of aqueous copper (II) sulphate solution using platinum electrodes.Ions present in solution:
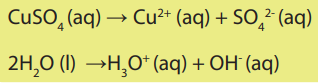
Cathode
The cathode gets coated with a brown layer of copper as the solution loses its blue
colour.
Anode

13.7.3. Concentration of electrolyte solution
Increase of concentration of an ion tends to promote its discharge, for example in
concentrated hydrochloric acid, containing
as negative ions, the highly concentrated is discharged in preference.
is discharged in preference.
However, if the acid is very dilute, some discharge of will also occur. It is important
will also occur. It is important
to know that as the acid is diluted, there will not be a point at which chlorine ceases
to be produced and oxygen replaces it. Instead a mixture of the two gases will comeoff, with the proportion of oxygen gradually increasing.
Another case in which the order of discharge according to the electrochemical series
is reversed by a concentration effect is that of sodium chloride solution.
In concentrated solution of sodium chloride called brine, the following reactions
occur.

Question 3
A university student set up three different electrolytic cells. The substances that
were electrolysed were NaCl (l), 0.05 M NaCl (aq) and 5.0 M NaCl (aq). Which of
the following statements correctly describes the results of the experiment?
a. The reactions occurring for the aqueous solutions will produce the same
products at the anode and cathode.
b. Chlorine gas is the major product when molten NaCl (aq) and 0.05 M
NaCl (aq) are electrolysed.
c. The pH at the cathode increases when solutions of NaCl are electrolysed.
d. The only means by which different products can be produced forvarying concentrations of NaCl is to alter the voltage.
13.7.4. The state of the electrolyte
The half reactions taking place at the electrode depends on whether the electrolyte
is in a molten or an aqueous state, and if in aqueous state its concentration. For
example, the electrode reactions that take place during the electrolysis of moltenpotassium iodide are:
However, if aqueous potassium iodide is used, the following electrode reactions
take place:
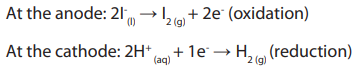
Electrode signs and reactions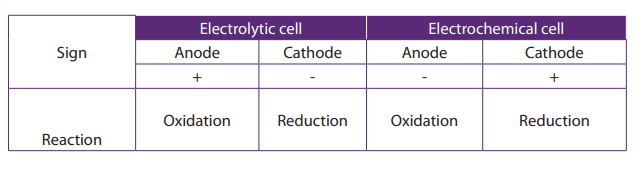
Checking up 13.7
Question 1
A student set up the following experiment. How would the rate of electrolysisin beaker 2 compare to that in beaker 1?
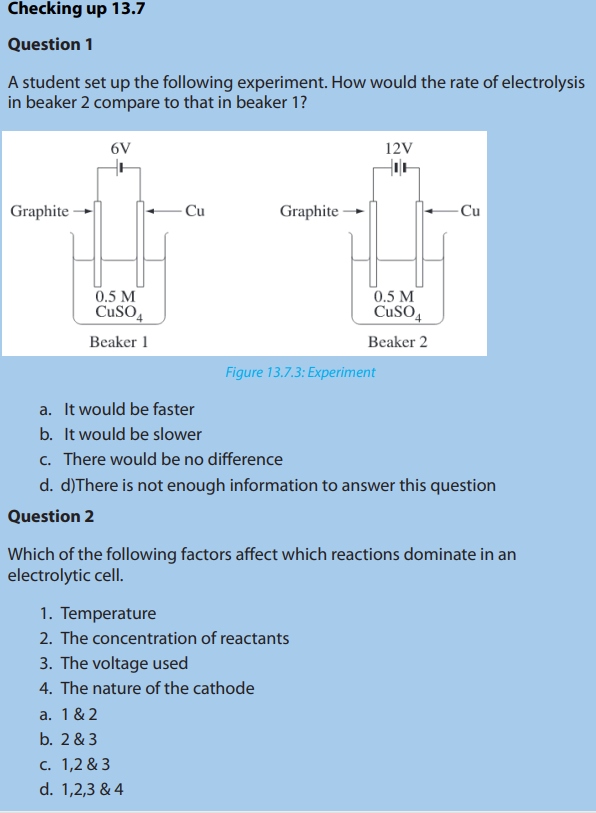
13.8. Application of electrolysis
Activity 13.8
Copper-Plated Key
Materials:
• 1.5-volt D battery with battery holder
• Two alligator clip leads or insulated wire
• Beaker or glass
• Copper sulphate
• Copper electrode (or coil of copper wire)
• Brass key• Safety equipment
Procedure:
5. Prepare the key for copper-plating by cleaning it with toothpaste or
soap and water. Dry it off on a paper towel.
6. Stir copper sulphate into some hot water in a beaker until no more willdissolve. Your solution should be dark blue. Let it cool.
3. Use one alligator clip to attach the copper electrode to the positive terminal
of the battery (this is now the anode) and the other to attach the key to the
negative terminal (now called the cathode).
4. Partially suspend the key in the solution by wrapping the wire lead loosely
around a pencil and placing the pencil across the mouth of the beaker. The
alligator clip should not touch the solution.
5. Place the copper strip into the solution, making sure it doesn’t touch the key
and the solution level is below the alligator clip. An electrical circuit has now
formed and current is flowing.
6. Leave the circuit running for 20-30 minutes, or until you are happy with the
amount of copper on the key.
Question: Observe carefully electrolysis process and records what happenedduring the electrolysis process.
Electrolysis has a number of important industrial applications. These include
the extraction and purification of metals, electroplating and anodizing and themanufacture of other chemicals for example sodium hydroxide (NaOH).
Extraction of metals
Metals in group I and II of the periodic table cannot be reduced by chemical
reducing agents; they are extracted from their fused halides by electrolysis. Sodium
is obtained by electrolysis of molten sodium chloride in the Dawncell. Magnesium is
obtained by electrolysis of genera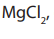 ted from dolomite and sea water.
ted from dolomite and sea water.
Extraction of aluminumThe chief ore of aluminum is bauxite,
 it contains silica and iron (III)
it contains silica and iron (III) oxide as impurities. Bauxite is dissolved in a strong solution of sodium hydroxide:
The impurities are not affected by the presence of sodium hydroxide because they
are not amphoteric and they are thus filtered off. The solution is diluted, cooled andseed by adding a few crystals of pure Al
On seeding, the aqueous tetrahydroxoaluminate is precipitated as pure Al

from the solution:

The electrolytic cell is an iron tank lined with carbon, which acts as the cathode. The
anodes are blocks of carbon dipped into the electrolyte. The electrolyte is a solution
of molten aluminum oxide in molten cryolite. Cryolite acts as a solvent to dissolve
aluminum oxide and as an impurity to lower the melting point of aluminum oxide.The electrolytic cell is maintained at around 900°C.
Aluminum ions are discharged at the cathode, forming a pool of molten aluminum
at the bottom of the tank.
At high temperature, oxygen reacts with the carbon anode to form carbon dioxide
gas. Hence, the anodes are slowly burnt away as carbon dioxide gas and needs to bereplaced frequently.
Manufacture of NaOH and extraction of
 gas in Downcell
gas in DowncellConstruction of Down’s cell
• Down’s cell consists of a rectangular container of steel.
• Inside of the tank is lined with firebricks.
• Anode is a graphite rod which projects centrally up through the base of the
cell.
• Cathode is a ring of iron, which surrounds the anode
The anode and cathode are separated from each other by a cylindrical steel
gauze diaphragm; so that and
and 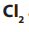 are kept apart.
are kept apart.• A bell like hood is submerged over the anode
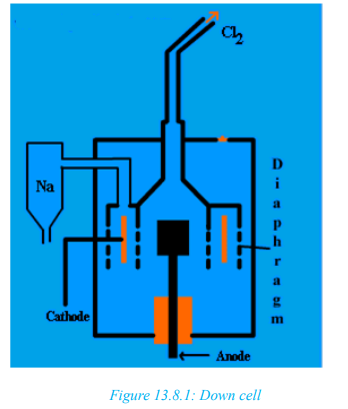

These are manufactured by electrolysis of concentrated sodium chloride, calledbrine. Hydrogen is also obtained as by products. In solution, sodium chloride ionizes:
Using carbon electrodes, the products of electrolysis are chlorine at the anode and
hydrogen at the cathode. Hydrogen is discharged in preference to sodium.
The sodium discharged at the mercury cathode forms a solution of sodium amalgam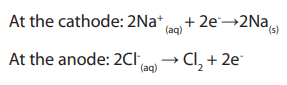
in mercury. The sodium amalgam is collected in a reservoir in which it reacts
with water to form sodium hydroxide solution and hydrogen gas. Mercury is alsorecovered and returned to the electrolytic cell to pass through the process again.
The sodium hydroxide produced is crystallized. It is used in: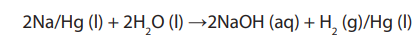
• Manufacture of soap
• The paper industry-wood contains lignin, which is a nitrogenous compound, in
addition to cellulose. Wood chips are converted into pulp by boiling the chips
with sodium hydroxide solution to remove the lignin. The digested material isbleached with chlorine.
Purification of metals
Metals such as copper, zinc and aluminium can be purified by electrolysis. The
purification of metals is known as refining. The copper obtained after the reduction
process is not very pure. It contains small amounts of impurities such as iron. This
copper is called blister copper and is refined by an electrolytic method. It is castinto bars which are used as anodes in acidified copper (II) sulphate solution.
The cathode is made of thin pure copper. During the electrolysis, ions are
ions are
transferred from the anode to the cathode where they are discharged and copper isdeposited.
The net effect is to dissolve the anode made of impure copper and thicken the
cathode (pure copper) with more pure copper.
Electroplating
This is a process of coating a metal with another of interest mainly to prevent it from
rusting, or/ and to improve its appearance, for example, in silver plating articles as
cake dishes, made of base alloy, for example cupronickel, are made the cathode in
plating bath of potassium (or sodium) dicyanoargentate(I), solution. This
solution. This
contains some silver ions, The anode is pure silver. When direct current passes,
The anode is pure silver. When direct current passes, the following reactions occur.
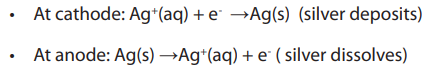
In general;
• The metal being coated is made the cathode and the metal coating is the anode
• The solution used is made of the ions of a metal that is coating, so that theanode can dissolve. Anode is the pure plating metal.
A good electroplating requires steady electric current, appropriate concentration of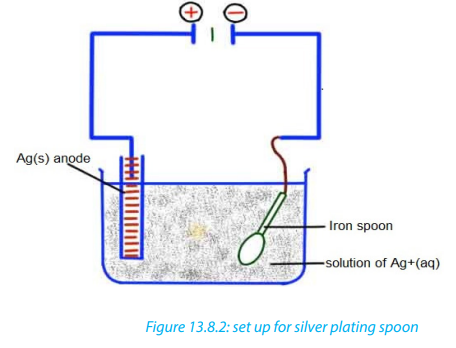
electrolyte and temperature. The metal to electroplate must be clean.
Checking up 13.8
1. a. What is the difference between electrolytic extraction of a metal and
electroplating?
b. Draw a set up used to electroplate a spoon by silver.
2.What is the material for cathode and anode during electro refining of impure
copper?
3. Electrometallurgy is the process of reduction of metals from metallic
compounds to obtain the pure form of metal. Given elements: aluminum,
lithium, sodium, potassium, magnesium, calcium, Zinc, Iron and copper
which ones can be reduced by chemical agents such as carbon and whichones are produced by electrolysis only?
13.9. End Unit Assessment
1. Choose from a list of words and fill in the missing words in the text below:
electrolysis, cathode and anode. When the current passes through an
electrolytic solution, ions migrate and electrons are gained or lost by ions
on the electrodes surface. Electrode that is positively charged has deficit
of electrons is called…………and other negatively charged has excess of
electrons is called……………. chemical changes at the electrodes due to the
passage of electric current are called ………………...
2. Answer by true or false
a. The Avogadro (1791-1867) did the first work on electrolysis and
formulated what is known today as faraday’s laws of electrolysis.
b. Electroplating is a process of coating a metal with another of interest
mainly to prevent it from rusting, and to improve its appearance.
c. A non electrolyte is a solution or molten compound which cannot be
decomposed by an electric current.
d. An ion is an atom or a group of atoms (radical) which has an electric
charge.
e. An anode is the negative electrode through which electrons leave
the electrolyte and a cathode is the positive electrode through which
electrons enter the electrolyte or leave the external circuit.
3. Which of the following involves electrolysis?
a. Photosynthesis and Respiration
b. Purification of Copper and Sea Water
c. Purification of copper and extraction of reactive metals
d. Extraction of reactive metals and Respiration
4. Which of the following is not an inert electrode?
a. Carbon
b. Copper
c. Platinum
d. Mercury
5. What ions are present in the electrolysis of aqueous Copper (II) Sulfate with
Copper electrodes?
a. Copper (II) ions, Sulfate ions
b. Hydrogen ions, Oxygen ions, Copper (II) ions
c. Copper (II)ions
d. Hydrogen ions, Hydroxide ions, Copper (II) ions, Sulfate ions
6. What happens during the electrolysis of a diluted sodium chloride solution?
a. Hydrogen ions and chlorine ions are discharged.
b. Hydrogen ions and hydroxide ions are discharged.
c. Sodium ion and chlorine ions are discharged.
d. Sodium ions and hydroxide ions are discharged.
7. State three applications of electrolysis on a large scale and describe one of
them briefly.
8. Describe how the factor of concentration affects electrolysis.
9. Calculate the amount of electricity required (in Faradays) to deposit one mole
of lead ions if a current of 2.5A was passed for 20 minutes through molten lead
(II) bromide and 3.20 g of lead metal was deposited.
10. An element X has a relative atomic mass of 88. When a current of 0.5A is
passed through the molten chloride of X for 32 minutes and 10 seconds, 0.44
g of X deposited at the electrode.
• Calculate the number of Faradays needed to liberate 1 mol of X.• Write the formula of the ion of X (1F= 96500C).
UNIT 14: ENTHALPY CHANGE OF REACTIONS
Key unit competency:
To be able to design an experimental procedure to verify the enthalpy
changes in a chemical reactionLearning objectives
• Define heat of reaction, standard enthalpy change of combustion, enthalpy
of neutralisation, enthalpy of solution, enthalpy of hydration and lattice
enthalpy
• Describe an experimental procedure in determination of heat of combustion
• Explain the relationship between quantity of heat produced and mass of
substance in combustion reaction
• State Hess’s law of constant heat summation
• State and explain the factors that affect the magnitude of lattice energy
• Describe bond breaking as endothermic and bond making as exothermic
• Develop practical experimental skills about enthalpy changes of reactions,
interpreting results and drawing valid conclusions.
• Carry out practical activities to determine enthalpy change of reactions
(enthalpy change of combustion of ethanol, enthalpy change of
neutralization).
• Calculate the enthalpy change of combustion, neutralization and dissolution
from experimental data
• Deduce how Hess’s law is applied to Born-Haber cycle.
• Construct Hess’s energy cycles and Born-Haber cycles from data obtained
experimentally or provided.
• Calculate the enthalpy changes of reactions using Hess’s law.
• Use the standard bond energy to determine the standard enthalpy of
reaction.
• Relate the heat of hydration and lattice energy to heat of solution.
• Respect of procedure during experiments of combustion and neutralization.
• Appreciate the contributions of other scientists such as Hess, Born andHaber’s work.
Introductory activity
Observe the pictures below, analyze them and answer the questions thatfollow.

Functioning of a vehicle’s engine Spacecraft launching Bunsen burner
1. What is the origin of the energy used for the flight of airplanes, the
functioning of vehicle’s engines or some machines used in factories,
launching of spacecrafts, energy used by our bodies, the energy
released by a burning wood, Bunsen burner or a burning match or an
exploding dynamite, etc?
2. What are the chemical reactions that are involved in the processes
above?3. How the energy used may be determined?
14.1. Definition of standard enthalpy of different reactions
Activity 14.1
Referring to the concept of energy changes and energy profile diagrams for
chemical reactions:
1. Recall the definition of:
a. enthalpy change
b. thermochemical equation
2. State the rules governing thermochemical equations.
3.By conducting your own research, differentiate the types of enthalpychange of reactions.
In thermodynamics, it is shown how energy, work, and heat are related. Every
chemical reaction occurs with a concurrent change in energy. Before to embark
the explanation of these chemical changes, some key terms have to be defined asfollows.
(i) Enthalpy change (ΔH)
In thermodynamics, the heat of reaction also known as enthalpy of reaction is
the change in the enthalpy (H) of a chemical reaction that occurs at a constant
pressure. Enthalpy, H is a state function used to describe the heat changes that
occur in a reaction under constant pressure. It is a state function as it is derived from
pressure, volume, and internal energy, all of which are state functions. The enthalpy
is a measurement of the amount of energy per mole either released or absorbed in
a reaction.
When a reaction is taking place in an open container, a quantity of heat which is
proportional to the quantity of the matter present, will be released or absorbed.
The flow of heat is the enthalpy change noted ΔH. The units of ΔH are kJ/mol or
kcal/mol.
(ii) Thermochemical equation
A thermochemical equation is a balanced equation that includes the amount ofheat exchanged (produced or absorbed).
Examples

The rules of enthalpy change of reaction:
a) The enthalpy change of a reaction is proportional to the amount of reactantsthat are involved in the reaction.
Examples:
b) In a chemical reaction, reversing a reaction automatically changes the sign of
∆H.
Example:

If the reaction is reversed, we have:
c) The enthalpy change of a reaction depends on the physical states of the
reactants and the products.
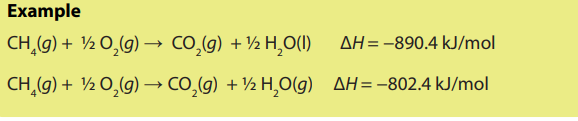
(iii) Types of enthalpy changes
There are various types of enthalpy change. Some examples of the types ofenthalpy changes are given below.
a) The enthalpy of formation of a substance is the heat change (heat released
of a substance is the heat change (heat released
or absorbed) for the chemical reaction in which one mole of the substance is formed
from its constituent elements under given conditions of temperature T and pressure
P.
The standard enthalpy of formation of a substance is the change in enthalpy
of a substance is the change in enthalpy
for the reaction that forms one mole of the substance from its elements in their most
stable form with all reactants and products at the pressure of 1 atm and usually atthe temperature of 298 K.
The standard enthalpy change of a reaction is the enthalpy change that occurs in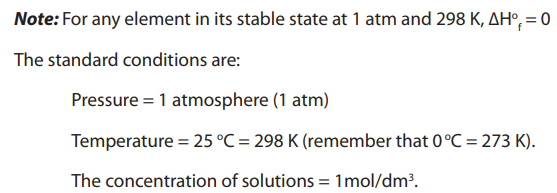
a system when matter is transformed by a given chemical reaction when all reactants
and products are taken in their standard states (at 1 atm and 298 K).
Consider a general reaction:
The standard enthalpy change for any reaction can be calculated from the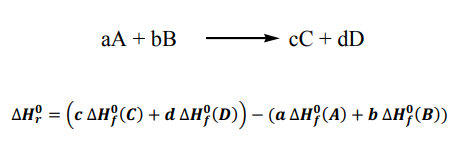
standard enthalpies of formation of the reactants and the products in the reaction:


Answer:

b) Enthalpy of combustion
The enthalpy of the combustion of a substance (element or compound) ΔHoc, is the
enthalpy change which occurs when one mole of a substance undergoes completecombustion with oxygen in excess at 298 K and 1 atm.
Examples:
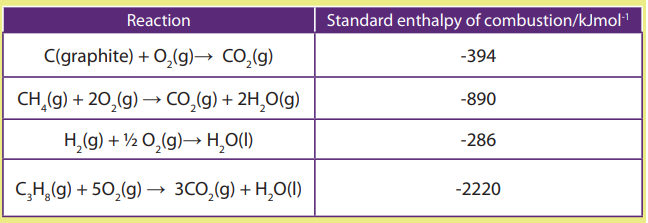
c) Enthalpy of neutralization
The standard enthalpy of neutralization, is the enthalpy change which occurs
is the enthalpy change which occurs
when one gram equivalent of an acid is neutralized by one gram equivalent of a
base to produce a salt and water under the standard conditions of temperature andpressure.
The equation of the neutralization reaction is:
Example:
Enthalpy change for the neutralisation of sodium hydroxide by hydrochloricacid is
The enthalpy of neutralization is the heat evolved for the reaction between the
ions given by the acid with the OH→ ions given by the base to form one mole of
d) Enthalpy of displacement
The displacement enthalpy is the enthalpy change of a reaction in which an
element displaces another in a chemical reaction.
For example, zinc is more reactive than copper, so when zinc is added to copper (II)sulphate solution, copper is displaced.
The reaction is associated with an enthalpy change converted into the heat energy
equals to

e) Enthalpy of solution
The enthalpy of solution of a compound is the heat energy change at constant
pressure when one mole of a compound is completely dissolved in a specific
amount of water as solvent.
However, if a large volume of the solvent is used till further addition of the solvent
does not produce any more heat change it is called enthalpy of solution at infinitedilution. The symbol (aq) is used to represent the solvent at large dilution.
Examples:
The enthalpy of solution of sodium chloride solid:

f) Enthalpy of atomization
The atomization enthalpy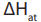 is the enthalpy change when one mole of gaseous
is the enthalpy change when one mole of gaseous
conditions. The enthalpy change of atomization is always positive.
For a diatomic molecule, the atomization enthalpy is equal to a half of the bonddissociation energy.
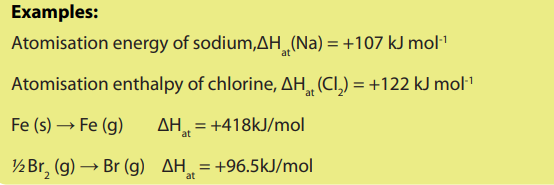
g) Lattice enthalpy
Lattice enthalpy is the enthalpychange that occurs when one mole of an ionic crystalisformed from its gaseous constituents.
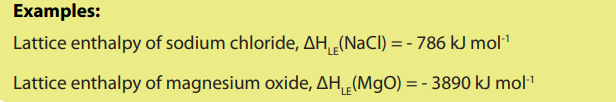
14.2. Relationship between temperature and heat
h) Hydration enthalpy
Hydration enthalpy is the enthalpy change when one mole of gaseous ions dissolvesin sufficient water to give an infinitely dilute solution.

i) Bond dissociation enthalpy
Bond dissociation enthalpy is the needed energy to break a single bond such as N-Hin an ammonia molecule.

Checking up 14.1
1. Write an equation representing the formation of each of the following
compounds from its constituent elements.
a. hexane
b. nitric acid
c. methanol
d. potassium bromide
e. butanoic acid
2. Which of the following reactions do not represent the standard enthalpyof formation?
3. Write the equations that represent the standard enthalpy of combustion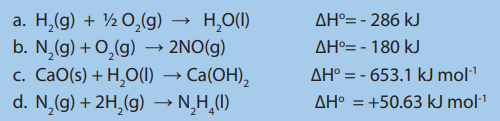
of:
a. hydrogen
b. methane
c. sulphur
d. propanol
4.Write equations for which the enthalpy of atomization is measured.
a. potassium
b. nitrogen
c. iodineActivity 14.2
To investigate the relationship between heat and temperature
Requirements
• Weighing balance
• Thermometer
• Insulated plastic beaker(calorimeter)
• Measuring cylinder
• Sodium hydroxide pellets• Distilled water
Procedure

Interpretation
Heat is the exchange of thermal energy between the system and the surroundings
that results in the temperature difference. Heat flows from matter with high
temperature to matter with low temperature until both objects reach the same
temperature. The quantity of heat is symbolized by “q”.
When a system absorbs heat its temperature increases. The increase in temperatureis directly proportional to the amount of heat absorbed.
The heat capacity, C of a substance is the amount of heat required to raise the
temperature of a substance by 1°C. The heat capacity is expressed in J/°C or J/K.
The molar heat capacity is the amount of heat energy required to raise the
temperature of one mole of a substance by 1°C. It is expressed in Joules per mole
per degrees Celsius (or Kelvin), (J/mol.°C or J/mol. K).
For example, the molar heat capacity of lead is 26.65J/mol.°C, which means that it
takes 26.65 Joules of heat to raise 1 mole of lead by 1°C.
The specific heat capacity is the amount of heat needed to increase the temperature
of one gram of a substance by one degree. It is expressed in Joules per gram per
degree Celsius (J/g.°C).Water has a very high specific heat, 4.18J/g.°C.
The high specific heat of water allows it to absorb a lot of heat energy without large
increase in temperature keeping ocean coasts and beaches cool during hot seasons.
It allows it to be used as an effective coolant to absorb heat.The relationship between heat and temperature is given by:
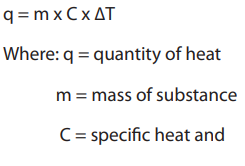
ΔT = change in temperature
Note: Heat capacity, C, can never be negative for a mass or a substance, and similarly
the specific heat of a substance can never be negative. Therefore, if the change in
temperature is negative, the initial temperature is higher than the final temperature.
The heat capacity of an object depends on its mass: 200 g of water requires twice as
much heat to raise its temperature by 1°C than 100 g of water. It also depends on the
type of material: 1000 J of heat energy will raise the temperature of 100 g of sand by12°C, but only raise the temperature of 100 g of water by 2.4°C.
14.3. Experimental methods for finding the standard enthalpy
of combustion reactions
Activity 14.3
To investigate the enthalpy of combustion of ethanol
Requirements
• spirit burner (containing ethanol)
• thermometer
• copper can
• measuring cylinder
• retort stand and accessories
• balance
• breeze shield
Safety precautions
• Ethanol is highly flammable and the main risk is from burns.
• Since a small amount is burned the build-up of any products of incomplete
combustion is negligible.
• Wear eye protection.
• Ensure the spirit burner is always sitting in a stable position.
• If you have to re-fill the spirit burner, allow it to cool and then fill it awayfrom sources of ignition.
Procedure
1. Weigh the spirit burner (already containing ethanol) with its cap on
and record its mass. (The cap should be kept on to cut down the loss of
ethanol through evaporation)
2. Using the measuring cylinder, measure out 100 cm3
of water into the copper can.
3. Set up the apparatus as directed by your teacher.
4. Measure and record the temperature of the water.
5. Remove the cap from the spirit burner and immediately light the
burner.
6. Slowly and continuously stir the water with the thermometer.
7. When the temperature has risen by about 10 °C, recap the spirit burner,
measure and record the maximum temperature of the water.8. Reweigh the spirit burner and record its mass.
Calculations
a. The heat energy gained by the water (q) can be calculated using the
formula: q = c m ∆T
b. The difference in the initial and final masses of the spirit burner gives us the
mass of ethanol burned (say x g) and so the heat energy we calculat is equal
to that released by burning x g of ethanol. It is assumed that all the heat
energy released by the burning ethanol is absorbed only by the water.
c. We can work out the mass of one mole of ethanol and knowing how much
heat energy is released when x g of ethanol is burned we can calculate the
heat energy released when one mole of ethanol is burned. This will be equalto the enthalpy of combustion of ethanol.
A calorimeter is a device used to measure the amount of heat energy exchanged
(released or absorbed) in a reaction. If a calorimetry experiment is carried out under
a constant pressure, the heat transferred provides a direct measure of the enthalpy
change of the reaction.
Simple determination of enthalpy of combustion can usea simple calorimeter described by the Figure 14.1below.
The energy produced by the combustion of the fuel is used to heat a known mass
(m) of water. Therefore, the heat provided by the fuel is equal to the heat receivedby water.
Its amount may be calculated using the relation:
Where: m is the mass of water
Cs is the specific heat capacity of water
∆T is the temperature changeKnowing the mass of the fuel used, its enthalpy of combustion may be calculated.
Example:

Answer:
A more accurate method of determining the enthalpy of combustion is the use of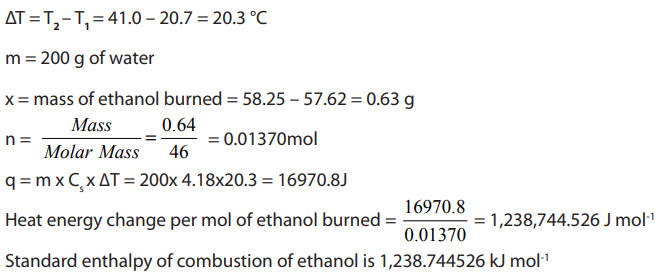
a bomb calorimeter (Figure 14.2). It is based on the same principle as the simple
experiment involving fuel burners, but it is more accurate because the heat loss isreduced to zero.
Figure 14.2 Schematic diagram of a bomb calorimeter
Source : https://www.chem.fsu.edu/chemlab/chm1045/energy.html
In a bomb calorimeter, a sample of a compound is electrically ignited and the heat
energy obtained by combustion heats the water in the calorimeter.
Checking up 14.3
14.4. Experimental methods for finding the standard enthalpy
of neutralisation reactions
Activity 14.4
To investigate the enthalpy of neutralization of hydrochloric acid by
sodium hydroxide.
Requirements:
Each group will be provided with:
2 plastic beakers
50 mL of 1 M HCl
50 mL of 1 M NaOH
Thermometer
ScaleWeigh boats
Procedure:

Discussion questions
Calorimetry is also used to determine the enthalpy change of a reaction taking
place in solution. For an exothermic reaction, the heat energy released increases the
temperature of the water in solution while for an endothermic reaction, the heat
energy absorbed is derived from water in the solution and the temperature of the
solution falls.
When hydrochloric acid reacts with sodium hydroxide, the temperature of the mixturerises and the heat is transferred to the plastic beaker, the process is exothermic.
Example:Consider the results from an experiment similar to the one described above:

Enthalpy change = mass of the solution x specific heat capacity x temperature rise

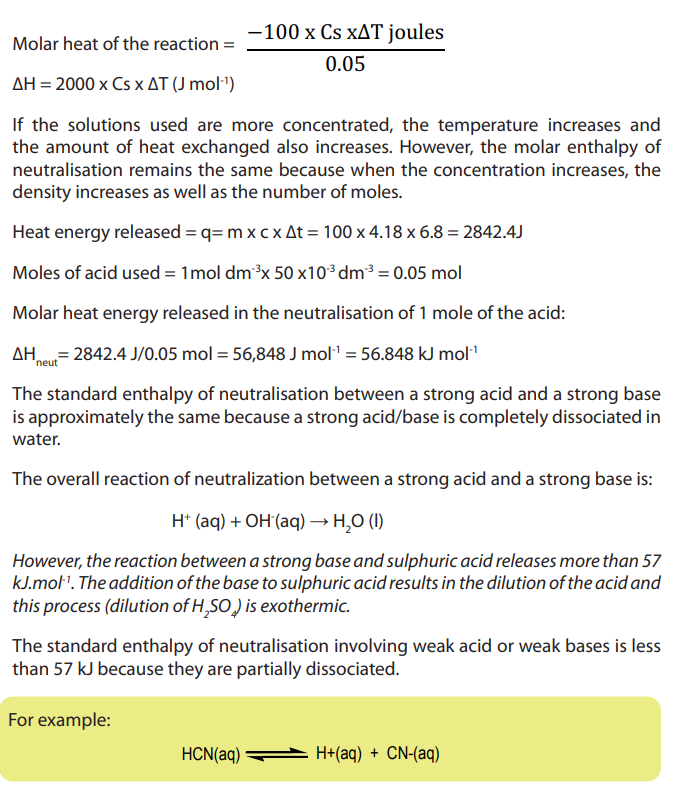

Checking up 14.4

14.5. Hess’s law or Law of constant heat summation
Activity 14.5
Observe the above image and answer the following questions.
1. In which case a person climbing by foot, Karisimbi volcano from point A to
point B:
a) Travels a greater distance?
b) Has more difficulties to reach point B?
2. In which case a person who climbs Karisimbi volcano from A to B, the gain in
gravitational energy is the highest?
3. Can you give a different example to illustrate the same phenomenon?
4. What conclusion to draw from the two examples?
5. Is any relation between the conclusion and the heat exchanged in a chemical
reaction? Illustrate your opinion with examples and come up with a general
conclusion.6. Compare your ideas to the first law of thermodynamics.
The increase in gravitational potential energy that occurs when a person climbs
from the base to the top of a volcano like Karisimbi or someone who is elevated
from the first to the fourth floor of a building is independent of the pathway taken.
That gain in gravitational potential is a state function that is analogous to a
thermodynamic state function.
Remember that the enthalpy (heat of reaction) is a state function. This means that a
change in enthalpy does not depend on how the change was made, but only on the
initial state and final state of the system; it is independent of the pathway.
In 1840, the Russian chemist Germain Henri Hess, a professor at the University of St.
Petersburg, discovered from his thermochemical studies that the enthalpy change is
a state function. The result from his experiment was known as Hess’s law or Law of
Constant Heat Summation. This law state that “the heat evolved or absorbed in a
chemical process is the same whether the process takes place in one or in several steps”
In other words, no matter how you go from a given set of reactants to a set of
products, the enthalpy change for the overall chemical reaction is the same whether
the reaction takes place in one step or in a series of steps.
The enthalpy change is independent of the pathway of the process and the number of
intermediate steps in the process.Hess’s law can be illustrated by the following reaction:
The reaction can be decomposed into two steps. The two steps and the overall
process are represented by the following thermochemical equations.
The two processes can be represented in a thermochemical cycle. This diagram is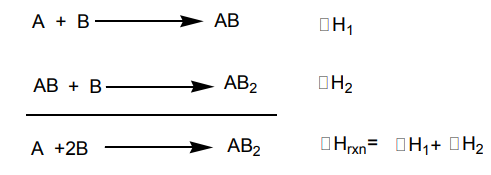
known as the Hess’s principle (Figure 14.3).
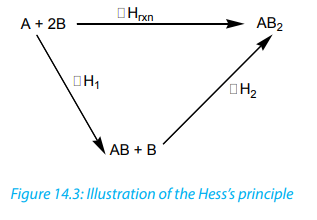
Examples:
1. Determination of the enthalpy change for the complete combustion of
carbon.
The direct reaction of carbon with oxygen yields carbon dioxide. However,
in the first step carbon gives carbon monoxide which is then oxidised
to carbon dioxide during the second step. The two-step reactions
corresponding to Hess’s law diagram for the formation of carbon dioxideare shown as follows.
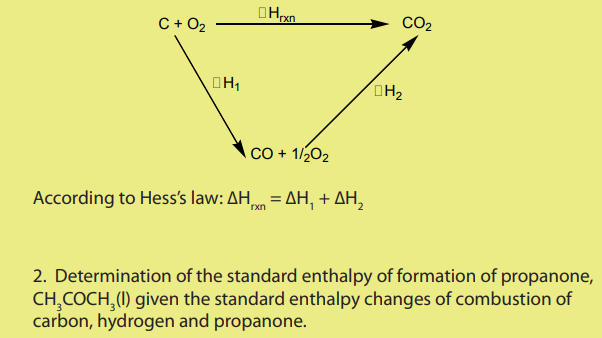
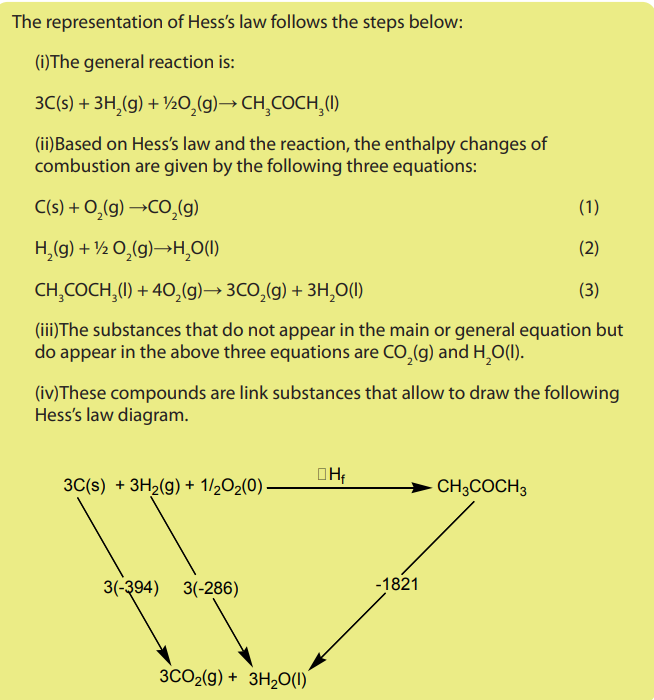
Note: The enthalpy change can be calculated by adding equations corresponding
to the different combustion to obtain the main equation. Then, the enthalpy change
is obtained by addition of the corresponding enthalpy change values multiplied byappropriate coefficient and if necessary, some of them are reversed.
Main equation:
Equation corresponding to the different combustions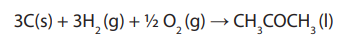
3 moles of C(s) react in the main equation so the first equation is multiplied by 3,
1 mole of propanone is formed in the main equation so the third equation should
be reversed.
Checking up 14.5
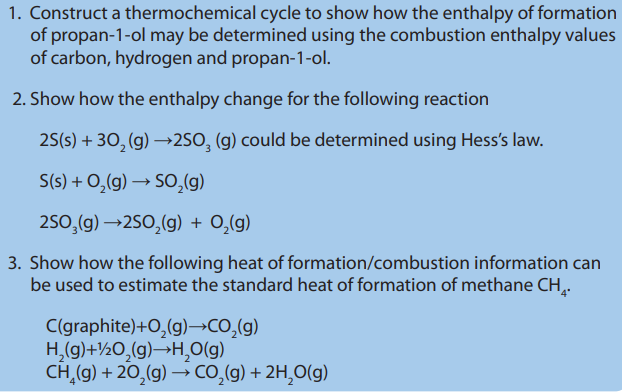
14.6. Application of Hess’s law
Activity 14.6
1. State the Hess’s law?
2. Describe how enthalpy of formation of propan-1-ol, hexane, sulphuricacid, can be determined.
The Hess’s law can be applied to calculate enthalpies of reactions that are difficultor impossible to measure.
Hess’s law states that if a reaction is carried out in a series of steps, ∆H of the
reaction will be equal to the sum of enthalpy changes for the steps provided
that the initial and the final conditions are the same. (Or the total enthalpy changeis independent of the route).
Examples:
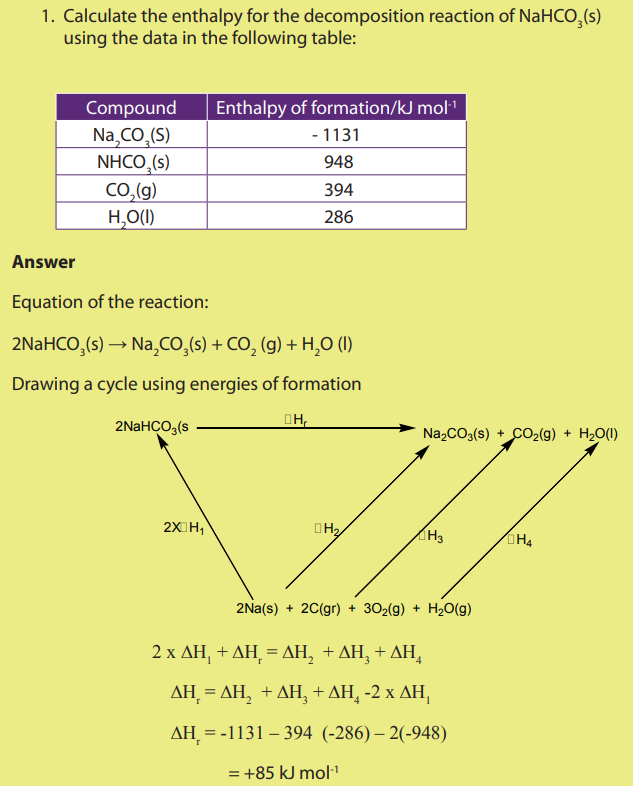


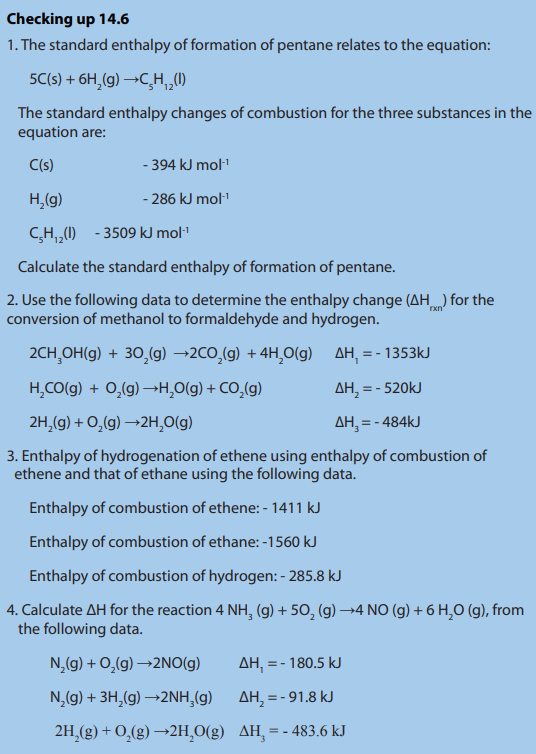
14.7. Born-Haber cycle
Activity 14.8
1. Refer to the trends in physical properties of chemical elements and
chemical bonding, explain each of the following terms.
a. Ionization enthalpy
b. Electron affinity
c. Atomization enthalpy
d. Dissociation enthalpy
e. Sublimation energy
f. Enthalpy of formation
2. State the Hess’s law.
3. Can the Hess’s law apply or not to the formation of an ionic compound?Justify your answer using an example.
In the previous sections, the Hess’s law and Lattice energy were discussed. Recall
that the Hess’s law of Constant Heat Summation states that the enthalpy change is
independent of the pathway of the process and the number of intermediate steps in theprocess.
Lattice formation enthalpy is the enthalpy change when one mole of an ionic
compound is formed from its gaseous ions at the standard temperature and pressure.
Because all the bonds in the ionic lattice are broken, it is an endothermic process.
To determine directly the lattice energy of an ionic solid experimentally is not
easier.
However, an indirect process known as Born–Haber cycle can be used based onHess’s law.
A Born-Haber cycle is a thermodynamic cycle which relates the lattice energy of
an ionic compound to its enthalpy of formation and other measurable quantities.
Lattice enthalpies of ionic compounds give a good indication as to the strength of
the ionic bonding in the lattice. Born-Haber cycle provides a useful way to account
for the relative stabilities of the chemical compounds and the relative stability of
the compound is determined by the lattice enthalpy of a compound. The lattice
enthalpy of an ionic compound is determined by breaking up the formation of an
ionic compound into a series of steps and then, all the steps will be added for the
overall reaction. In general, Born-Haber cycles are enthalpy cycles that show howionic compounds are formed from their elements.

14.8. Lattice enthalpy
Activity 14.7
Referring to what you have learned so far in chemistry, attempt the following questions.
1. What is meant by ionic bond?
2. Explain the main steps in the formation of an ionic bond showing clearlythe energy change involved in each step.
A lot of energy is released as the ionic bond is formed. The reaction is highly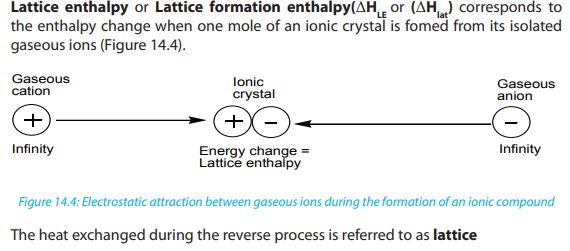
exothermic because there are strong electrostatic attractions between ions of
opposite charges. The relative values of lattice enthalpy are governed by the chargedenssity of the ions.
The value of lattice formation enthalpy cannot be measured directly; it is calculated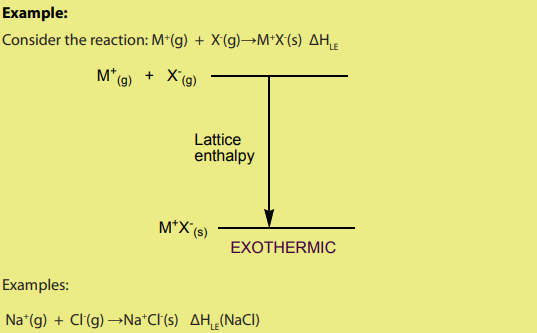
using the Born-Haber cycle. The greater the charge densities of the ions, the morethey attract each other and the larger the lattice enthalpy.
The more exothermic the lattice enthalpy, the higher is the melting point.
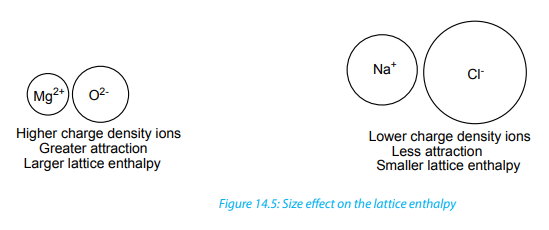
dissociation enthalpy.
Lattice dissociation enthalpy is the enthalpy change when one mole of an ionic
lattice dissociates into isolated gaseous ions. Since there is a strong electrostatic
attraction between ions of opposite charge, a lot of energy must be supplied toovercome the attraction.
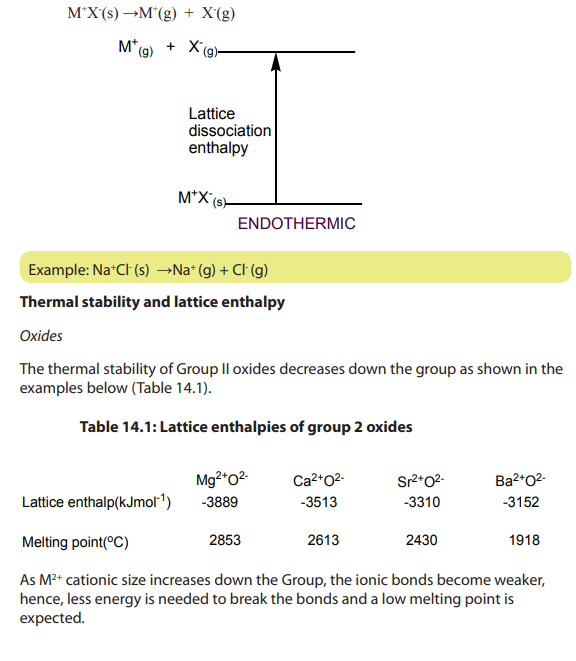

Examples of enthalpy changes that are commonly used in Born-Haber cycles are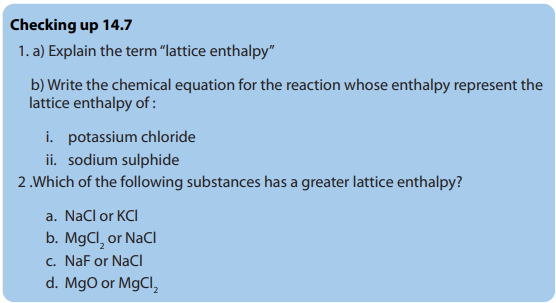
the following:
i. Enthalpy change of formation
ii. Atomisation enthalpies
iii. Ionisation enthalpyiv. Electron affinity
The following diagram represents a general illustration of the Born-Haber cycle.
The application of Hess’s law to this cycle made possible the calculation of the
lattice enthalpy.
The arrows pointing upwards represent the endothermic changes while those
pointing downwards show the exothermic changes.
From the Born-Haber Cycle, the enthalpy change associated with the route depicted
by the red arrow is equal to the enthalpy change of the route shown by the bluearrow.



Checking up 14.8
1. Construct and interpret a Born-Haber cycle for calcium fluoride showing
clearly the enthalpy changes involved.
2. a. Write an equation for the standard enthalpy of formation of aluminium
oxide.
b. Construct and interpret a Born-Haber cycle for aluminium oxide and
explain how it would be used to calculate the lattice enthalpy of aluminiumoxide.
14.9. Born-Haber cycle: Calculations of the lattice enthalpy
Activity 14.9
1. Construct a Born-Haber cycle for the formation of an ionic compound MX.
2. Explain how the cycle can be used to calculate the lattice enthalpy ofthe compound mentioned in 1)
Examples:
1. Determining the lattice enthalpy of lithium fluoride using the Born-Haber
cycle
Using the Born-Haber cycle constructed previously for lithium fluoride, itslattice enthalpy can be calculated as follow

2. Determination of the first electron affinity of oxygen
A Born-Haber cycle for the formation of calcium oxide is shown below.
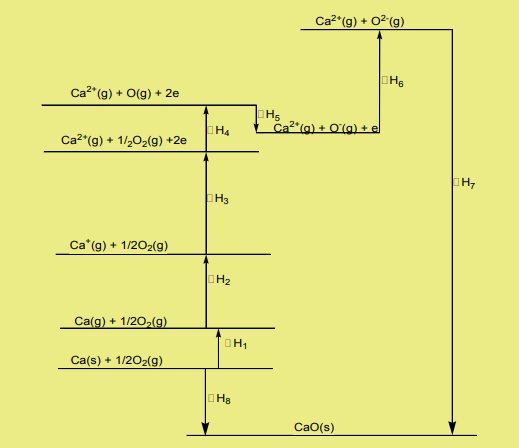
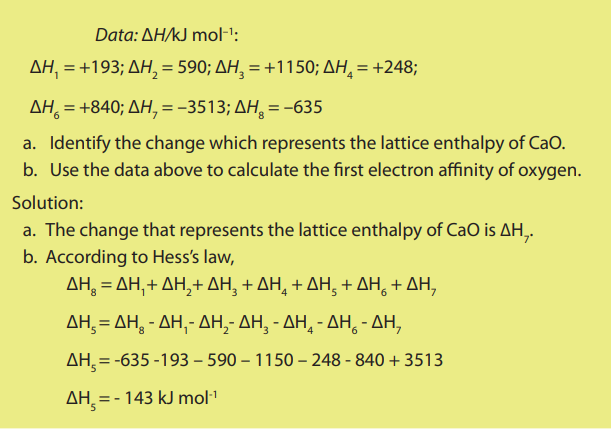

14.10. Hydration energy
Activity 14.10
Experiment on solubility of ionic compounds
Requirements:
Test tubes
Spatula
Distilled water
Sodium chloride
Sodium hydroxide
Potassium bromide
Silver chloride
Sodium carbonate
Potassium hydroxide
Magnesium hydroxide
Magnesium sulphate
Barium hydroxide
Barium sulphate
Procedure
1. Put 10mL of distilled water in a test tube
2. Add a half spatula end full of sodium chloride and shake
3. Record all your observations
4. Repeat steps 1-3 using the different salts5. Record your findings in the table below.
6. Compare the lattice enthalpy of compounds of soluble compounds to
those of sparingly soluble or insoluble compounds and deduce the general
trends.7. What term is given to the interactions between solute and solvent?
Some ionic compounds such as sodium hydroxide and sodium chloride are very
soluble while others like magnesium carbonate, are sparingly soluble or insoluble
(calcium carbonate, magnesium hydroxide, barium sulphate). The dissolution of
some compounds such as sodium hydroxide release the heat energy and the processis exothermic.
If a pair of oppositely charged gaseous ions are placed together, they are attracted
to each other. The energy change (lattice enthalpy) is highly exothermic. If the ions
were placed in water, they would be attracted to the polar water molecules leading
to the energy change (hydration enthalpy) which is highly exothermic. In both cases,the greater the charge density of the ions, the more exothermic will be the process.
The enthalpy change when one mole of a gaseous ion dissolves in water (excess) to
give an infinitely dilute solution is called enthalpy change of hydration
The solvent-solute interactions are referred as “solvation”.
When an ionic compound dissolves in water, the process can either be exothermic
or endothermic.
The enthalpy of solution of a compound is the heat energy change at constant pressure
when one mole of a compound dissolves completely in water.
For example when sodium chloride dissolves in water, the overall process isrepresented as:
The first step is to separate the ions in the crystal. This requires energy to overcome
the attractive forces between oppositely charged ions. The corresponding latticedissociation energy according to the dissociation of the compound is:
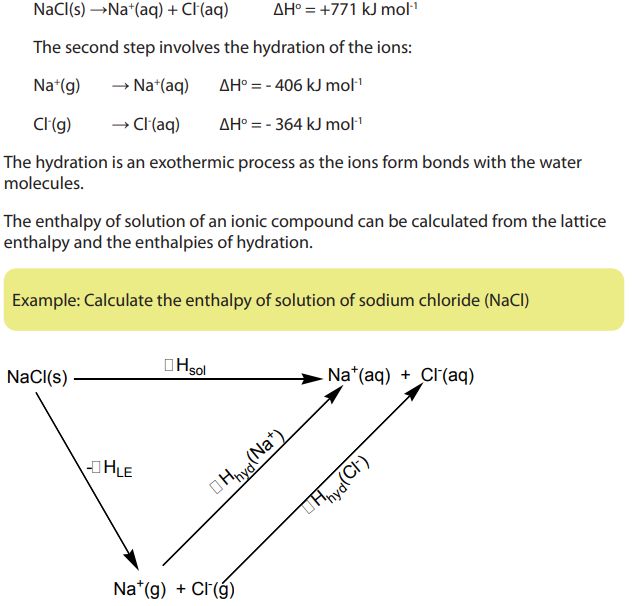

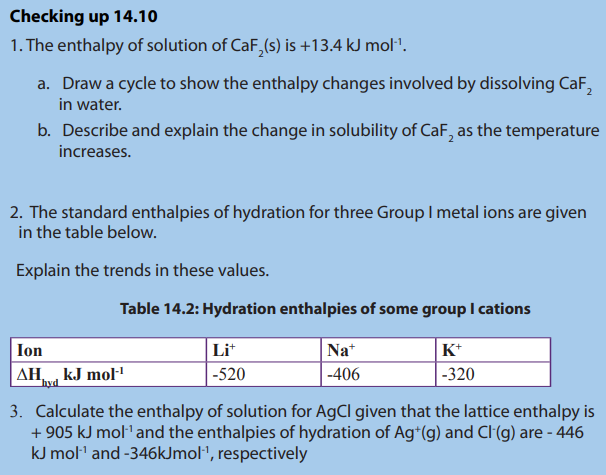
14.11. Average standard bond enthalpy
Activity 14.11
Using internet or textbooks do research and answer the following questions.
1. Explain the term covalent bond and describe the formation of a covalent
bond.
2. What are the factors that affect the strength of a covalent bond?3. Relate the bond strength to the reactivity of a covalent compound
1. Formation of a covalent bond
The covalent bond is a bond formed when atoms share a pair of electrons to
complete the octet. The covalent bonds mostly occur between non-metals or
between molecules formed by the same (or similar) elements. Two atoms with
similar electronegativity do not exchange an electron from their outermost shell;the atoms instead share electrons and their valence electron shell is filled.
The build-up of electron density between two nuclei occurs when a valence atomic
orbital of one atom overlaps with that of another atom, each orbital containing a
single electron.
The orbitals share a region of space, i.e. they overlap. The overlap of orbitals allows
two electrons of opposite spin to share the common space between the nuclei,forming a covalent bond.
2. Factors influencing the strength of a covalent bond
i. Size of the atoms
Small atoms have shorter bond length and thus have better overlap of
orbitals while
larger atoms tend to have more diffused orbitals, resulting in less effective
overlap
ii. Number of bonds between atoms
Bond strength: triple bond > double bond > single bond
“>”: “stronger than”
Species with more electrons to share form more bonds between atoms,
resulting in atoms held closer together.Polarity of bon
iii) Polar bond is generally stronger than non-polar bond; the extra
electrostatic attraction between partial charges gives rise to a stronger bond.
iv. Presence of neighbouring lone pair electrons
Atoms which are very small in size and have lone pairs in close proximity will
result in excessive repulsion that weakens the covalent bond.
3. Bond enthalpy
Bond enthalpy (energy) is the amount of energy required to break one mole
of gaseous bonds to form gaseous atoms. It is known as the bond dissociation
enthalpy.
Bond energy may also be defined as the amount of energy released when two
atoms are linked together by a covalent bond. It is expressed in kJ mol-1 or Kcalmol-1.
The bond energy is a measure of the strength of a chemical bond. The smaller
the bond enthalpy the weaker the bond and the easier is to break the bond.
The process of breaking a bond is endothermic while the bond-formation is anexothermic process.
Note: For diatomic gas molecules, the bond enthalpy is equal to two times the
enthalpy of atomization.
The exact bond enthalpy of a particular chemical bond depends on the environment
of the bond in the compound. Therefore, the bond enthalpy values given areaveraged values (Table 14.3).
Table 14.3: Examples of some values of average bond enthalpy.
The bond enthalpy values are used to:
• compare the strengths of bonds
• estimate the enthalpy change of a reaction
• explain the mechanisms of reaction• explain the structure and bonding

14.12. Calculating enthalpy change of reaction using average
bond enthalpies
Activity 14.12
1. Define the term average bond enthalpy.2. Consider the following reactions:

a. Explain how the enthalpy change of each of them may be determined.
b. During chemical reactions, bonds are broken in the reactants and
new bonds are formed in the products. When bonds are broken, there
is a release of heat energy known as the bond dissociation energy.
When bonds are formed energy is released. Do research and show the
relationship between average bond dissociation enthalpy values and the
heat exchanged during a chemical process.
Breaking chemical bonds requires energy (endothermic), and forming chemical
bonds releases energy (exothermic). To determine if a chemical reaction is
endothermic or exothermic depends on whether or not breaking the old chemical
bonds requires more energy than the energy released from the new chemical bondsformed.
When the standard enthalpy change for a reaction cannot be measured, an
approximate value is obtained by using average standard bond enthalpies. During
a chemical reaction, the energy is provided to break bonds of the reactants, and
energy released when the new bonds of the products are formed.
The standard enthalpy of reaction is the difference between the sum of average
bonds enthalpies of the products and the sum of average standard bond enthalpiesof the reactants.



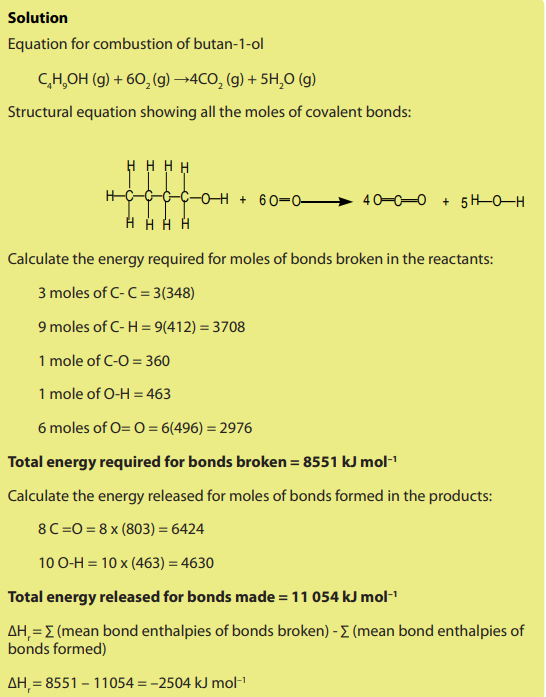

14.13. End unit assessment

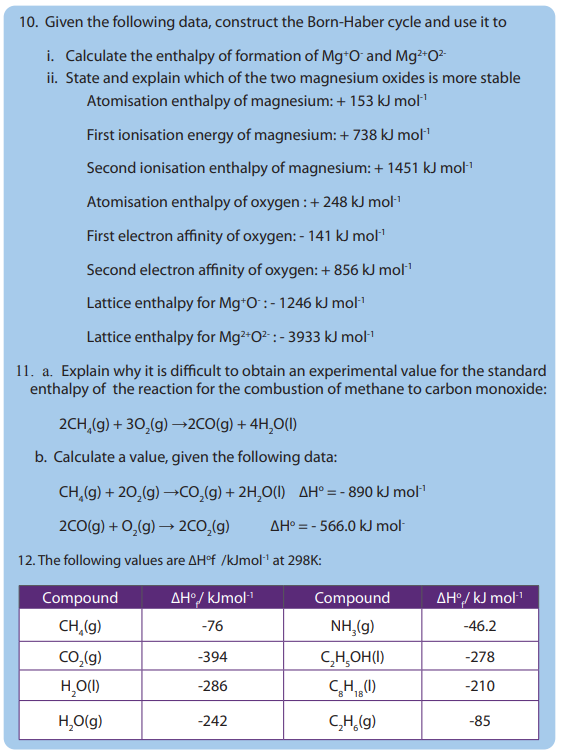


UNIT 15: ENTROPY AND FREE ENERGY
Key unity competence:
To be able to Predict the feasibility of chemical reactions.
Learning Objectives
By end of this unit a learner should be able to:
• Explain the term entropy
• State the second law of thermodynamics
• State if the value of free energy for a reaction will be positive or negative.
• Relate the entropy changes to the changes in degree of disorder• Predict the spontaneity of reactions using the Gibbs free energy values
INTRODUCTORY ACTIVITY
Observe the pictures above of playing cards and answer related questions.
1. What is the difference between the two organizations of playing cards
2. In which conditions (arrangement) to play card is easy? Explain3. Why people toss cards before playing them
15.1. Definition of entropy and change in entropy
Activity 15.1 Investigation of entropy of a substance.
1. Take three beakers and label them by A, B, and C
2. In beaker A add 50 grams of ice
3. In beaker B add 50 mL of water
4. In beaker C add 25 mL of water and heat it up to transformation to liquid
state (vapors)
5. Pour the container of beaker A and B, then compare it to the movement
of vapors in C
a. In which beaker, A, B or C where the molecules move easily (high
speed)?
b. In which beaker A, B or C where the molecules move slowly (low
speed)?
c. Order the beakers to the order of mobility of the container.
d. Explain why the mobility of molecules in beakers A, B and C varies.
e. Which factor that make the variation of water in different states tomove differently in the beakers A, B and C?
15.1.1. Definition of entropy
Entropy is a thermodynamic function that describes the number of arrangements
(positions and/or energy levels) that are available to a system existing in a given
state. Entropy is a quantitative measure of microscopic disorder for a system. It is defined
as the degree of disorder or randomness of a system. The entropy of a system increases
as the disorder of the system increases. When we focus on the molecular motion of a
system, adding heat to this system increases the disorder because the heat increasesthe randomness of the molecular motion. So, the entropy of the system increases.
The effect of adding heat to a system increases the molecular motion, and this
results in more disorder of the system. Entropy is derived from the second law of
thermodynamics, which states that “all systems tend to reach a state of equilibrium”.
Based on the state of matter, the entropy increases in the order: Solids < liquids < gases
substances (Figure 15.1). If we consider three substances such as solid, liquid and a gas.
A mole of a substance has a much smaller volume in the solid state than it does in
the gaseous state. In the solid state, the molecules are close together, with relatively
few positions available to them; in the gaseous state, the molecules are far apart,
with many more positions available to them. The liquid state is closer to the solidstate than it is to the gaseous state in these terms.
In the above Figure, the molecules are closely packed in the solid state, in the liquid
state; the molecules are not very closed while in a gas the molecules are far apart
which increase the entropy in gas than liquid or solid.
The following Tables 15.1 and 15.2 show the relationship between the state of asubstance and its entropy:
Table 15.1. Entropy variation with state of a substance
In the above Table 15.2, the element argon has a greater entropy value than the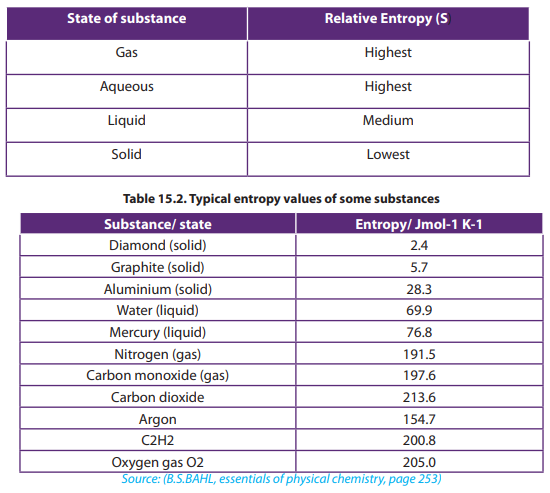
carbon element (graphite) because argon is a gas with greater disorder and random
particle movement than the solid state of carbon.
The entropy of a perfect crystal at absolute zero is exactly equal to zero. At absolute
zero (zero kelvin), the system must be in a state with the minimum possible energy.
As the temperature increases, the particles vibrate more and the disorder increases.
Melting is associated with an increase in entropy (disorder), however, boiling
is associated with a large increase in entropy: gases are associated withconsiderable random particle in movement and disorder.
15.1.2. Change in entropy
Entropy, like temperature, pressure, and enthalpy, is also a state property and is
represented in the literature by the symbol “S”. The entropy change is represented
by ΔS.
It is known that the main purpose of chemistry is the study of chemical reactions. In
this section, it is important to consider the entropy changes accompanying chemical
reactions that occur under conditions of constant temperature and pressure. The
entropy changes in the surroundings are determined by the transfer of heat that
occurs when the reaction takes place. However, the entropy changes in the system
(the reactants and products of the reaction) are determined by positional probability.For example, consider the reaction of production of ammonia in the Haber process:
It is seen that four molecules of reactants yield two molecules of ammonia product
which lead to less disorder in the system. If the number of molecules of the gaseous
products is greater than the number of molecules of the gaseous reactants, positional
entropy typically increases, and ∆S will be positive for the reaction.The calculation of the entropy change of a reaction is given by applying the formula:
For a chemical reaction which involves the reactants and the products, the change
in entropy is calculated as follows.
Any reaction that results in the formation of a gas, an increase in the number of
gaseous moles, results in the increase of the disorder. Entropy change, ∆S, relates to
increasing disorder of a process, either arising through physical change (e.g. melting,
evaporation) or chemical change (e.g. dissolution, evolution of
from hydrogen carbonates with acid) or both.

The chemical reactions are favored if they are accompanied by an increase in entropy.
2. Consider the thermal decomposition of solid calcium carbonate, predict the signof the standard entropy

In this reaction, a solid reactant produces a molecule of gas, the positional entropy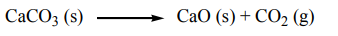
increases, and
 is positive
is positive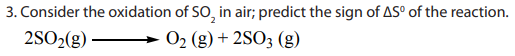
For this reaction, three molecules of gaseous reactants become two molecules of
gaseous products. Because the number of gas molecules produced decreases, the
entropy decreases also, and
 is negative.
is negative.Note:
i. Many endothermic reactions proceed spontaneously under normal
conditions because there is an increase in entropy.
ii. Some exothermic reactions do not proceed spontaneously because there is
a decrease in entropy. In a system with perfect order, the entropy is equal to
zero. An example of perfect order is found in a crystalline substance at the
absolute zero of temperature, where atomic and molecular motion cease.The entropy of pure, perfect crystal can be taken to be zero at 0 K.
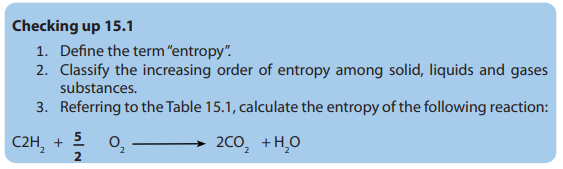
15.2. Second law of thermodynamics
Activity 15.2
1. State and explain the first law of thermodynamics
2. What is the relation between the first law of thermodynamics andthermochemistry?
In chemistry, thermodynamics deals with the energy and work of a system. There
are three laws in thermodynamics: first, second and third law. The first law establish
the relationship between the different forms of energy present in a system (kinetic
and potential), the work done by the system and the energy or heat transferred. The
second law of thermodynamics is dealing with entropy which describes the disorder
of the system. The Second law of thermodynamics states that in any spontaneous
process, the state of entropy of the entire universe, as an isolated system always
increases over time. It also states that the changes in the entropy in the universe cannever be negative.
Following are the statements of second law of thermodynamics:
a. All spontaneous process are irreversible in nature.
b. The net entropy of the universe in any natural process always increases
and tends to acquire the maximum value.
c. In a reversible process, the sum of entropies of the system and surroundings
remains constant but in an irreversible process, the total entropy of the systemand surroundings increases.


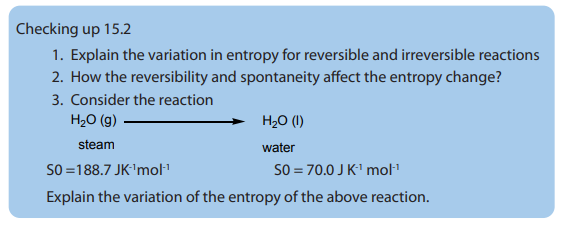
15.3. Free energy, the deciding factor
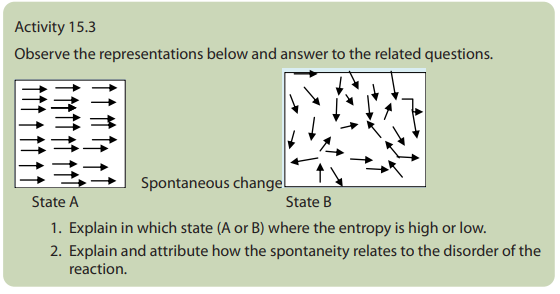
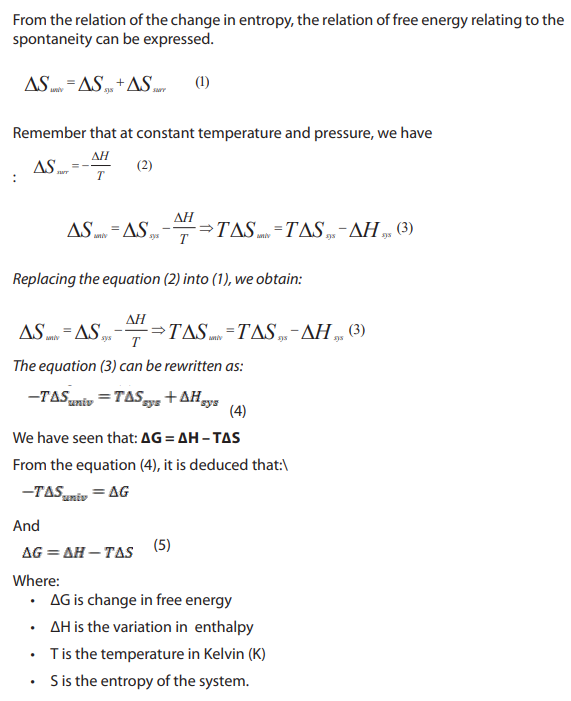

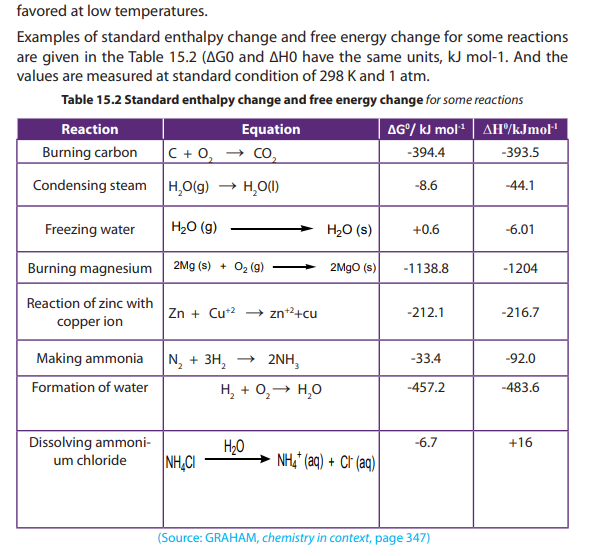
 1. Define exothermic and endothermic reaction
1. Define exothermic and endothermic reaction15.4. Feasibility of chemical reactions: relationship between free
energy, enthalpy and entropy feasibility
Activity 15.4.
2. What is the relation between enthalpy change and entropy or the
reaction?
3. Explain how a reaction is favored by the entropy.4. How the entropy and enthalpy make the reaction to be possible

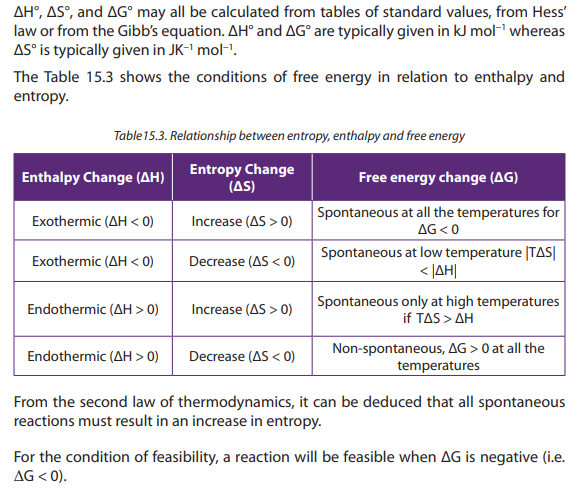
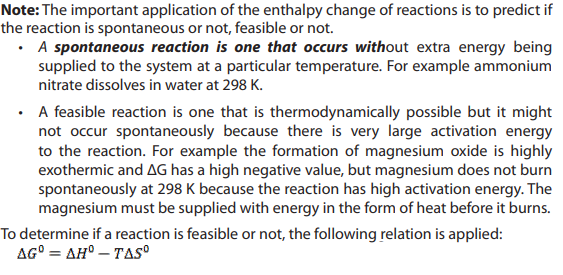
Answers: here we refer to the relation between the entropy free energy and
enthalpy
a. negative because both ∆H and (-T∆S) are negative
b. could be negative or positive because ∆H is negative and (-T∆S) is
negative
c. could be negative or positive because ∆H is negative and (-T∆S) is
positive
d. positive because both (-T∆S) and ∆H are positive
e. in (a) no, ∆H and(-T∆S) both are negative at all temperatures
f. In (b) yes at high T (-T∆S) has high negative value and may have a positive
∆H
In (c) yes, negative ∆H could be positive (-T∆S) at high temperature
In (d) no, ∆H and (-T∆S) are both positive at all temperatures


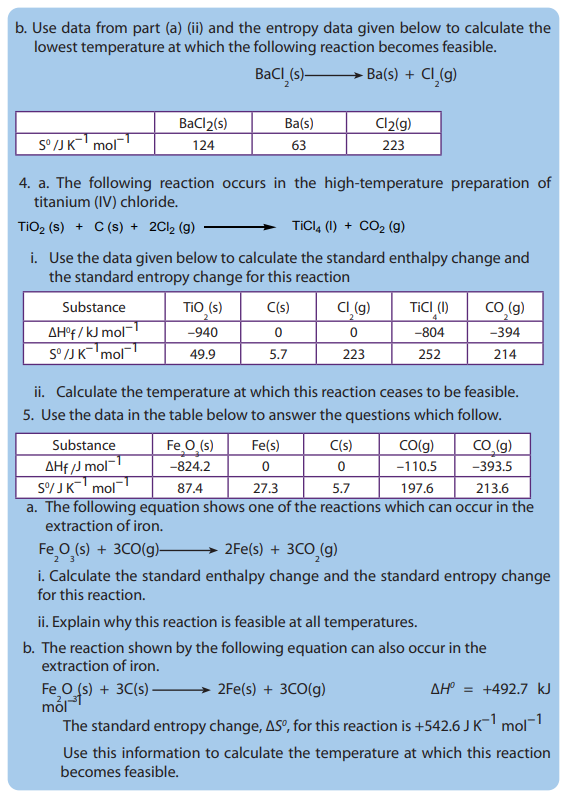
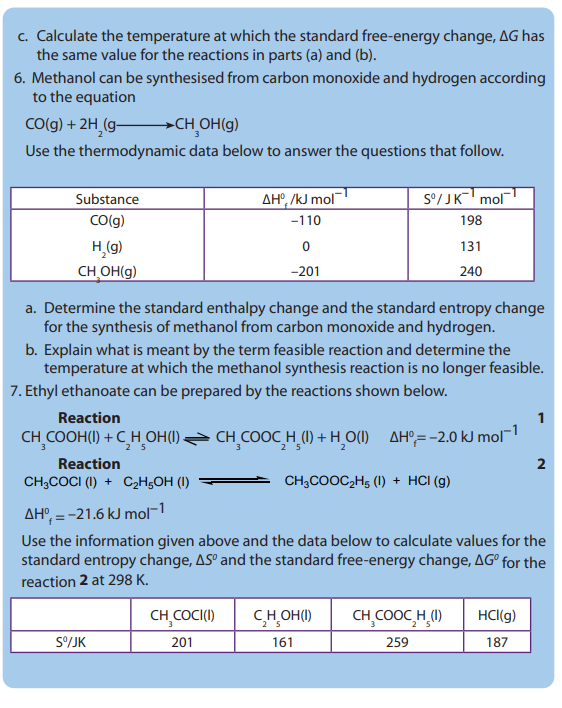
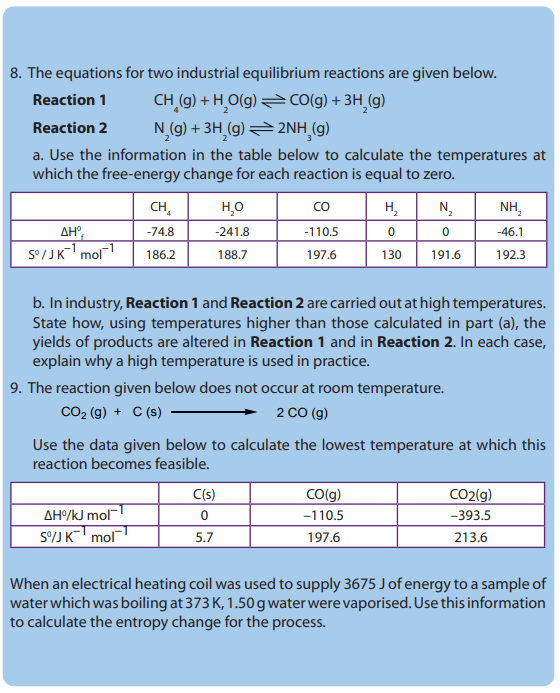
REFERENCES
Chang, R. (2005). Chemistry, Eighth Edition. New York: McGraw-Hill.
Dakin, H., & West, R. (1928). A general reaction of amino acids. II. Journal of
Biological Chemistry, 745-756. Retrieved Mars 25, 2018, from http://www.jbc.org/
content/78/3/745.citation
Schmitz, A. (2018, March 25). Introduction to Chemistry: General, Organic,
and Biological (v. 1.0). Retrieved from 18.1 Properties of Amino Acids:
https://2012books.lardbucket.org/books/introduction-to-chemistry-general
organic-and-biological/s21-01-properties-of-amino-acids.html
Kazemi, F., Kiasat, A.R. and Mombaini, B. (2007). Simple preparation of
symmetrical carboxylic acid anhydrides by means of Na2CO3/SOCl2. An
International Journal for Rapid Communication of Synthetic Organic Chemistry,
37(18): 3219-3223
E.N.Ramsden. (2000). A-level chemistry. United Kingdom: Nelson Thornes Ltd.
Holman, G. H. (1995). Chemistry in context. UK: Thomas Nelson and sons Ltd.
Petr Cann, P. H. (2002). Chemistry for advanced level. London: John Murray Ltd.
Daniel R.Bloch (2012), Organic chemistry DeMYSTiFieD, second edition,
McGrawHill.
Graham Hill, J. H. (2000). Chemistry in context,fifth edition. Nerson Thornes.
Obot, K. (2003). Organic chemistry for Advanced level.
Rwanda Education Board. (2015). Advanced level chemistry syllabus. Kigali:
PRINTEX.
Bruice, P. Y. (n.d.). Organic Chemistry fourth Edition.
COLLIN, A. (2008). Advanced organic chemistry: exercises,eighth edition.
D.Mukama, S. a. (2013). Chemistry for Rwanda Secondary Schools,Advanced
Level Senior5. Fountain.
Silver Obonyo, D. M. (2013). Chemistry for Secondary Schools. Fountain .
http://www.zygia.com/study/book ;
www.markedbyteachers.com
www.citycollegiate.com
